




|
STUDENT DIGITAL NEWSLETTER ALAGAPPA INSTITUTIONS |

|
Victor M. Ilizaliturri, Jr., MD
Being later steps in the supply chain heart attack meme order inderal 40mg with amex, distribution and utilization need to be discussed in the context of earlier decision points heart attack arena 10mg inderal for sale, such as cultivation and harvesting arterial occlusion discount 80 mg inderal with amex. In turn heart attack prevention discount inderal 80mg on-line, these logistics through end-use issues influence siting arrhythmia multiforme discount 80 mg inderal visa, scalability pulse pressure with exercise generic inderal 40 mg with visa, and the ultimate economics and operations of an integrated algal biofuels refinery. As a variety of fuel products-ethanol, biodiesel, higher alcohols, pyrolysis oil, syngas, and hydroreformed biofuels-are being considered from algal biomass resources, the specific distribution and utilization challenges associated with each of these possible opportunities is discussed. In all cases, biofuels infrastructure costs can be lowered in four ways: · Minimizing transport distance between process units Maximizing material energy density and stability Maximizing compatibility with existing infrastructure. Ethanol, biodiesel, biogas, renewable gasoline, diesel, and jet fuels are all possible products from algal biomass. Some of these fuels appear to be more compatible with the existing petroleum infrastructure (Figure 9. Specifically, jet-fuel blends from a variety of oil-rich feedstocks, including algae, have been shown to be compatible for use in select demonstration flights (Buckman 2009; Efstathiou and Credeur 2009). It is also anticipated that gasoline and diesel range fuels from algae will not require significant distribution system modifications during or after processing in the refinery. With more than 10 billion gallons per year produced and consumed domestically, distribution-related issues for ethanol have been studied for some time, and algal ethanol can benefit from these analyses. While not as energy-dense as purely petroleum-derived fuels, ethanol is an important fuel oxygenate that can be used in regular passenger vehicles and special flex-fuel vehicles at up to 10% and 85% gasohol blends, respectively. However, considerable infrastructure investments need to be made for higher ethanol blends to become even more attractive and widespread. One issue is that ethanol is not considered a fungible fuel; it can pick up excessive water associated with petroleum products in the pipeline and during storage, which causes a phase separation when blended with gasoline (Wakeley et al. One possible way to address this is to build dedicated ethanol pipelines; however, at an estimated cost of $1 million/mile of pipeline, this approach is not generally considered to be economically viable (Reyold 2000). Another possibility is to distribute ethanol blends by rail, barge, and/or trucks. Trucking is currently the primary mode to transport ethanol blends at an estimated rate of $0. This amount is a static number for low levels of ethanol in the blends (5%15%); as the ethanol content in the blend increases, the transport costs will also increase due to the lower energy density of the fuel. While the demonstration flights mitigate some infrastructure concerns, other distribution aspects concerning algal biomass, fuel intermediates, and final fuels remain poorly studied: · the stability of the algal biomass under different production, storage, and transport scenarios is poorly characterized, with some evidence suggesting that natural bacterial communities increase the rate of algae decomposition (Rieper-Kirchner 1990). In the context of a variety of culturing and harvesting conditions differing in salinity, pH, and dewatering levels, it is difficult to predict how these factors will influence biomass storage and transport, as well as the quality of the final fuel product. An issue impacting oleaginous microalgae feedstocks is that the transport and storage mechanisms of algal lipid intermediates have not yet been established. It is conceivable that these "bio-crudes" will be compatible with current pipeline and tanker systems. However, it is known that the presence of unsaturated fatty acids causes auto-oxidation of oils (Miyashita and Takagi 1986), which carries implications for the producers of algae and selection for ideal lipid compositions. It is also known that temperature and storage material have important implications for biodiesel stability (Bondioli et al. Thus, · materials and temperature considerations similar to plant lipids may be possibly taken into account for the storage of algae lipids (Hu et al. A significant factor restricting distribution of algal biomass as an intermediate is the amount of water it contains. Even for dewatered algal biomass, this will increase the mass to be transported by an order of magnitude and present corrosion issues. Drying presents concerns in terms of greenhouse gas emissions (see chapter 5); therefore, more research is needed, for example, on small-scale hydrotreatment. Depending on whether it will be dewatered/densified biomass and/or fuel intermediates that are to be transported to the refinery, conforming to existing standards. Because of the variability and instability of algal biomass, the distribution system will require novel monitoring and control. The optimal transport method(s) should be analyzed and optimized for energy inputs and costs, within the context of where the algae production and biorefinery facilities are to be sited. Petroleum refiners have shown remarkable flexibility in producing fit-for-purpose fuels from feedstocks ranging from light crude to heavy crude, oil shales, tar sands, gasified coal, and chicken fat, and are, thus, key stakeholders in reducing the uncertainty about the suitability of algal feedstocks for fuel production. The failure of a fuel to comply with even one of the many allowable property ranges within the prevailing specifications can lead to severe problems in the field. Comparison of Typical Properties of Petroleum Oil and Oil from Fast Pyrolysis of Wood and Microalgae. Research and technology advancements may one day yield optimized conversion processes, which can deliver algae-derived compounds with improved performance, handling, and environmental characteristics relative to their petroleum-derived hydrocarbon counterparts. If significant benefits can be demonstrated, new specifications can be developed. Additionally, some customers (such as airlines who face intensive scrutiny for their environmental impacts) may require the assurance of an objective, third-party, sustainability standard, such as that provided by the Roundtable on Sustainable Biomaterials. The discussion below is divided into separate sections that deal with algal blendstocks to replace gasoline-boiling-range and middle-distillate-range petroleum products, respectively. These classifications were selected because the compounds comprising them are largely distinct and non-overlapping. Within each of these classifications, hydrocarbon compounds and oxygenated compounds are treated separately, since their production processes and in-use characteristics are generally different. The primary algae-derived blendstocks that are suitable for use in this product range are biodiesel (oxygenated molecules) and renewable diesel (hydrocarbon molecules). The known and anticipated end-use problem areas for each are briefly surveyed below. Biodiesel has been demonstrated to be a viable fuel for compression-ignition engines, both when used as a blend with petroleum-derived diesel and when used in its neat form (i. The oxidative-stability and cold-weather performance issues of biodiesel preclude it from use as a jet fuel. The anticipated issues with algaederived biodiesel are similar, with added potential difficulties including: 1) contamination of the esters with chlorophyll, metals, toxins, or catalyst poisons. Hydrocarbons: Renewable Diesel and Synthetic Paraffinic Kerosene the hydrocarbon analog to biodiesel is renewable diesel, which is a non-oxygenated, paraffinic fuel produced by hydrotreating bio-derived fats or oils in a refinery (Aatola et al. However, hydrocarbons derived from algae are likely to be blended into petroleum fuels. The straight-chain hydrocarbons will provide a significant cetane enhancement, which can be exploited in refinery blending systems, which will also improve the cold-flow properties. The degree of saturation of the algal lipid is an important factor in the economics of hydrotreatment and also in the transportation stability of intermediates. Research is needed regarding how to optimize algal lipids intended for hydrotreatment. Also, as was the case with algal biodiesel, contaminants and end-product variability are concerns. Algal Blendstocks for Alcohol and Gasoline-Range Petroleum Products While much of the attention paid to algae is focused on producing lipids and the subsequent conversion of the lipids to diesel-range blending components (discussed above), algae are already capable of producing alcohol (ethanol) directly, and there are several other potential gasoline-range products that could be produced by algae-based technologies and biorefineries. Petroleum products in the alcohols and gasoline range provide the major volume of fuels used by transportation vehicles and small combustion engines in the United States. Ethanol or butanol is the most common biofuel currently used in gasoline, and these alcohols can be produced from fermentation of starches and other carbohydrates contained in algae. Additionally, the hydrotreating of bio-derived fats or oils in a refinery will typically yield a modest amount of gasolineboiling-range hydrocarbon molecules. The fuel and engine co-optimization effort includes two thrusts: Thrust I Improve near-term conventional spark ignition engine efficiency. This engine platform, which includes kinetically controlled and low-temperature combustion approaches, offers the promise of significantly greater thermal efficiencies with lower criteria-pollutant emissions, and presents attractive options for both light- and heavy-duty vehicles. Resources and Sustainability the development and scale-up of algal biofuels production, as with any biomass-based technology and industry, needs to be analyzed from a resource availability and sustainability perspective. To achieve success regarding both technical and economic performance without adverse environmental impacts, resource factors must be appropriately matched to the required growth conditions of the algal species being cultivated and the engineered growth systems. The sustainability of algal production systems can be evaluated using a system of social, environmental, and economic indicators. Evaluation of these indicators for sustainability assessments of algal biofuel production will be affected by local siting and resources considerations. Resource assessment modeling, as well as technoeconomic and life-cycle analyses, are discussed in chapter 11. This chapter provides an overview of the key resources and sustainability requirements for microalgae production and the progress that has been made in addressing these needs. These factors and parameters are of greatest importance to siting, facilities design, production efficiency, and costs. For each parameter, a variety of conditions may be more or less cost-effective for the siting and operation of algal biomass production. Additional resources include materials, capital, labor, and other inputs associated with facilities infrastructure and conducting operations and maintenance. High-level overview of the algal biofuel supply chain, illustrating key resource inputs and related environmental issues spanning the operations of biomass production through downstream processing and conversion to fuels and co-products. Many of the findings of these earlier assessments still apply today, and the potential remains for biofuels and other co-products derived from photoautotrophic microalgae to significantly contribute to meeting U. An in-depth summary of the resource assessment modeling work completed for algal biofuels production can be found in chapter 11. In addition to coastal and inland photoautotrophic microalgae production, off-shore marine environment concepts have been proposed. The integration of wind and solar energy on coastal and inland photoautotrophic microalgae cultivation sites has been proposed (Nair and Paulose 2014; Beal et al. High-level illustration of heterotrophic and photoautotrophic approaches to microalgal biomass and biofuels production (Source: Adapted from the 2010 National Algal Biofuels Technology Roadmap. The algae are cultivated in the dark in closed industrial bioreactors that could potentially be established in many locations throughout the country. Achieving affordable scale-up and successful commercial expansion using the heterotrophic approach relies on the cost-effective availability of organic carbon feedstock-a resource that ultimately links back to a photosynthetic origin. Heterotrophic and photoautotrophic approaches to microalgae production have different siting and resource input implications, and thus present synergistic integration opportunities, but are not discussed in this review. Mixotrophic cultivation systems combine photoautotrophic and heterotrophic processes and have a range of resource requirements that are determined by the scale of production (Table 10. Heterotrophic production can be characterized as more of an industrial operation with a significant upstream logistics trail associated with the sourcing of the needed biomass-derived input feedstocks, whereas photoautotrophic production, in terms of cultivation and harvesting, is more akin to agriculture and serves as the point of origin for the biomass feedstock supply for the downstream value chain. Resource issues for the heterotrophic approach are more largely associated with the upstream supply of organic carbon feedstock derived from commodity crops, selected organic carbon-rich waste streams, and lignocellulosic biomass, thereby sharing many of the same feedstock supply issues with first- and second-generation biofuels (Table 10. Use of sugars from cane, beets, other sugar crops, and from the hydrolysis of starch grain crops can lead to the problem of linking biofuel production and competition with food and feed markets. The preferred source of sugars and other appropriate biogenic carbon feedstocks for sustainable heterotrophic algae production are carbon-rich waste streams and the successful deconstruction of lignocellulosic materials. This work includes evaluation of siting and resource availability issues that are closely aligned with the production, availability, supply logistics, and pretreatment of lignocellulosic biomass feedstock that is expected to be capable of national scale-up to more than one billion tons annually (Perlack et al. Further discussion on the different approaches to microalgal biomass cultivation and production is in chapter 4. Sustainability Indicators for Photoautotrophic Microalgae Biofuels In addition to identifying the availability of resources for algal cultivation, assessments are also important to guide the responsible stewardship of resources toward environmental and socioeconomic sustainability. Sixteen largely quantitative, indicators of environmental sustainability have been proposed for algal biofuels (Table 10. These environmental sustainability indicators are categorized under soil quality, water quality and quantity, greenhouse gases, biodiversity, air quality, and productivity (Efroymson and Dale 2015). Proposed socioeconomic sustainability indicator categories are social well-being, energy security, external trade, profitability, resource conservation, and social acceptability (Table 10. Throughout the chapter, the sustainability indicators will be described as they pertain to different resources for algae cultivation. Sunlight and Temperature Needs Growth of algae is technically feasible in many parts of the United States, but the availability of adequate sunlight and the suitability of climate and temperature are key factors that will determine economic feasibility. Availability of abundant sunlight is important for both photoautotrophic microalgae growth in open and closed cultivation systems. The average seasonal insolation is generally the dominant and rate-limiting factor for autotrophic algal productivity, and this factor varies widely across the country among inland, coastal, and offshore sites. The daily, seasonal, and annual variation in solar insolation, as well as other climate-related factors, such as temperature and weather (cloud cover, precipitation, wind, etc. Resource Conservation Demonstrate high percent favorable opinion Show a progressively increasing or high value Show a progressively increasing or high value Frequency of catastrophic events based on current incidence or similar technology In general, the optimal temperature for algal biomass growth is between 2035°C (68-95°F), though strains vary in temperature tolerance. Colder temperatures can lead to slower growth and productivity rates, while hotter temperatures can potentially reduce productivity rates or even cause the death of individual alga (Pate 2013). As a result, lower latitude areas are preferred for a more stable temperature range (Pate et al. The operating temperature range used for algae biomass production can be altered through the use of 10. Evaporative water loss, pond depth, pond mixing, solar gain during the day, radiative heat loss at night, and the thermal coupling and bidirectional heat flow through pond bottom and walls affect optimum tempature conditions in open ponds. Temperature and availability of sunlight, both seasonally and annually, will most directly affect productivity, whereas precipitation, evaporation, and severe weather will affect water demand and water quality in open systems. Additional factors could conceivably help producers overcome what might otherwise be unfavorable climate conditions for algae production. This could include situations where co-location of microalgae production might be possible with industrial operations capable of providing excess heat and power for cost-effective environmental control of algal cultivation (Khawam et al. This scenario, however, requires a more refined analysis for systems that are likely close and highly integrated with co-located industries providing synergistic opportunities for utilizing waste heat and energy. Seasonal Considerations A critical climate issue for both open and closed cultivation systems is the length of economically viable growing season(s) for the particular strains of algae available for productive cultivation. The primary geographical location drivers for determining length of growing seasons are latitude and elevation, which have major influence on the hours and intensity of available sunlight per day and the daily and seasonal temperature variations. While some analyses have been conducted and/or reported on seasonal variability (Davis 2012; Venteris et al.
After centrifugation hypertension medical definition purchase inderal 40 mg with mastercard, the separated organelles are identified by detection of marker enzymes in the sample heart attack 86 years old buy inderal 10mg on-line. Nucleus is surrounded by two membranes: the inner one is called perinuclear membrane with blood pressure instrument buy inderal 10 mg overnight delivery. Typical cell 1= Nuclear membrane; 2= Nuclear pore; 3= Nucleolus; 4= endoplasmic reticulum; 5= Golgi body; 6= Mitochondria; 7= Microtubule; 8= Lysosome; 9= Vacuole; 10= Plasma membrane hypertension prevalence discount 80 mg inderal mastercard. Nucleus 8 Textbook of Biochemistry; Section A: Chemical Basis of Life numerous pores arterial thrombosis order inderal 40 mg free shipping. In some cells heart attack belanger remix buy inderal 80mg overnight delivery, a portion of the nucleus may be seen as lighter shaded area; this is called nucleolus. It is a network of interconnecting membranes enclosing channels or cisternae, that are continuous from outer nuclear envelope to outer plasma membrane. Under electron microscope, the reticular arrangements will have railway track appearance. Microsomal cytochrome P-450 hydroxylates drugs such as benzpyrine, aminopyrine, aniline, morphine, phenobarbitone, etc. The rough appearance is due to ribosomes attached to cytoplasmic side of membrane where the proteins are being synthesized. Newly synthesized proteins are sorted first according to the sorting signals available in the proteins. Then they are packed into transport vesicles having different types of coat proteins. Finally, they are transported into various destinations; this is an energy dependent process. Golgi complex 35,00040,000 x g, 30 min Microsomes Cytoplasm 75,000100,000 x g, 100 min Supernatant 4. They are formed into a secretory vesicle, where these products are stored for a longer time. Release of trypsinogen by pancreatic Chapter 2; Subcellular Organelles and Cell Membranes 9 Table 2. Catalase and peroxidase are the enzymes present in peroxisomes which will destroy the unwanted peroxides and other free radicals. These crystals when phagocytosed, cause physical damage to lysosomes and release of enzymes. Following cell death, the lysosomes rupture releasing the hydrolytic enzymes which bring about postmortem autolysis. Cathepsins are normally restricted to the interior of lysosomes, but certain cancer cells liberate the cathepsins out of the cells. Then cathepsins degrade the basal lamina by hydrolysing collagen and elastin, so that other tumor cells can travel out to form distant metastasis. There are a few genetic diseases, where lysosomal enzymes are deficient or absent. Silicosis results from inhalation of silica particles into the lungs which are taken up by phagocytes. This stimulates fibroblast to proliferate and deposit collagen fibers, resulting in fibrosis and decreased lungs elasticity. Inclusion cell (I- cell) disease is a rare condition in which lysosomes lack in enzymes, but they are seen in blood. This means that the enzymes are synthesized, but are not able to reach the correct site. It is shown that mannose-6phosphate is the marker to target the nascent enzymes to lysosomes. Mannose-6-phosphatedeficient enzymes cannot reach their destination (protein targetting defect). Discovered in 1950 by Rene de Duve (Nobel prize 1974), lysosomes are tiny organelles. Endocytic vesicles and phagosomes are fused with lysosome (primary) to form the secondary lysosome or digestive vacuole. As long as the lysosomal membrane is intact, the encapsulated enzymes can act only locally. But when the membrane is disrupted, the released enzymes can hydrolyse external substrates, leading to tissue damage. Polysaccharide hydrolysing enzymes (alpha-glucosidase, alpha-fucosidase, beta-galactosidase, alphamannosidase, beta-glucuronidase, hyaluronidase, aryl sulfatase, lysozyme) b Protein hydrolysing enzymes (cathepsins, collagenase, elastase, peptidases) c. Nucleic acid hydrolysing enzymes (ribonuclease, deoxyribonuclease) 10 Textbook of Biochemistry; Section A: Chemical Basis of Life. The inner mitochondrial membrane contains the enzymes of electron transport chain (Chapter 19). The fluid matrix contains the enzymes of citric acid cycle, urea cycle and heme synthesis. Cytochrome P-450 system present in mitochondrial inner membrane is involved in steroidogenesis (Chapter 46). This leads to formation of empty peroxisomes or peroxisomal ghosts inside the cells. Primary hyperoxaluria is due to the defective peroxisomal metabolism of glyoxalate derived from glycine (Chapter 15). The integral inner membrane proteins, are made by mitochondrial protein synthesising machinery. Antibiotics inhibiting bacterial protein synthesis do not affect cellular processes, but do inhibit mitochondrial protein biosynthesis (Chapter 41). Taking into consideration such evidences, it is assumed that mitochondria are parasites which entered into cells at a time when multicellular organisms were being evolved. These parasites provided energy in large quantities giving an evolutionary advantage to the cell; the cell gave protection to these parasites. This perfect symbiosis, in turn, evolved into a cellular organelle of mitochondria. The lipid bilayer shows free lateral movement of its components, hence the membrane is said to be fluid in nature. However, the components do not freely move from inner to outer layer, or outer to inner layer (flip-flop movement is restricted). Gerd Binning and Heinrich Rohrer introduced the scanning electron microscopy in 1981 by which the outer and inner layers of membranes could be visualized separately. It has highly selective permeability properties so that the entry and exit of compounds are regulated. The cellular metabolism is in turn influenced and probably regulated by the membrane. Membranes are mainly made up of lipids, proteins and small amount of carbohydrates. Phospholipids are the most common lipids present and they are amphipathic in nature. Fluid Mosaic Model the lipid bilayer was originally proposed by Davson and Danielle in 1935. Later, the structure of the biomembranes was described as a fluid mosaic model (Singer and Nicolson, 1972). The phospholipids are arranged in bilayers with the polar head groups oriented towards the extracellular side and the cytoplasmic side with a hydrophobic core. The distribution of the phospholipids is such that choline containing phospholipids are mainly in the external layer and ethanolamine and serine containing phospholipids in the inner layer. When cholesterol concentration increases, the membrane becomes less fluid on the outer surface, but more fluid in the hydrophobic core. The effect of cholesterol on membrane fluidity is different at different temperatures. At temperature below the Tm cholesterol increases fluidity and there by permeability of the membrane. In spur cell anemia and alcoholic cirrhosis membrane studies have revealed the role of excess cholesterol. The decrease in membrane fluidity may affect the activities of receptors and ion channels. Fluidity of cellular membranes responds to variations in diet and physiological states. The nature of the fatty acids also affects the fluidity of the membrane, the more unsaturated cis fatty acids increase the fluidity. The fluidity of the membrane is maintained by the length of the hydrocarbon chain, degree of unsaturation and nature of the polar head groups. Cis double bonds create a kink in the hydrocarbon chain and have a marked effect on fluidity. Similarly the fusion and budding of viral particles are also mediated by caveolae. Anchoring of proteins to lipid bilayers: Several peripheral membrane proteins are tethered to the membranes by covalent linkage with the membrane lipids. Since the lipids are inserted into the hydrophobic core, the proteins are firmly anchored. A typical form of linkage is the one involving phosphatidyl inositol which is attached to a glycan. This glycan chain includes a glucose covalently attached to the C terminus of a protein by the ethanolamine and to the phosphatidyl inositol by the glucosamine. They are areas on the membrane having predominantly glycosphingolipids and cholesterol. The localization and activity of the protein can be regulated by anchoring and release. Lipid rafts have a role in endocytosis, G protein signaling and binding of viral pathogens. Membrane proteins may be anchored by covalent bonding, palmitoylation and myrystoylation. Caveolae are flask shaped indentations on the areas of lipid rafts that are involved in membrane transport and signal transduction. The integral membrane proteins are deeply embedded in the bilayer and are attached by hydrophobic bonds or van der Waals forces. Some of the integral membrane proteins span the whole bilayer and they are called transmembrane proteins. The hydrophobic side chains of the amino acids are embedded in the hydrophobic central core of the membrane. The transmembrane proteins can serve as receptors (for hormones, growth factors, neurotransmitters), tissue specific antigens, ion channels, membrane-based enzymes, etc. Bacterial Cell Wall Prokaryotic (bacterial) cells as well as plant cells have a cell wall surrounding the plasma membrane; this cell wall provides mechanical strength to withstand high osmotic pressure. This tight junction permits calcium and other small molecules to pass through from one cell to another through narrow hydrophilic pores. Absence of tight junction is implicated in loss of contact inhibition in cancer cells (Chapter 51). They contain specialized proteins such as occludin, claudins and other adhesion molecules. The carrier molecules exist in two conformations Chapter 2; Subcellular Organelles and Cell Membranes 13 chromosomal movements during cell division. Molecular Motors Proteins that are responsible for co-ordinated movements in tissues and cells are referred to as molecular motors. Water channel or aquaporin Myelin Sheath It is made up of the membrane of Schwann cells (Theodor Schwann, 1858) condensed and spiralled many times around the central axon. Myelin sheaths thin out in certain regions (Node of Ranvier) (Anotoine Ranvier, 1878). Due to this arrangement, the propagation of nerve impulse is wavelike; and the speed of propagation is also increased. Upon stimulation, there is rapid influx of sodium and calcium, so that depolarization occurs. The ions flow in and out of membrane only where membrane is free of insulation; hence the wave-like propagation of impulse. In multiple sclerosis, demyelination occurs at discrete areas, velocity of nerve impulse is reduced, leading to motor and sensory deficits. Microvilli Microvilli of intestinal epithelial cells and pseudopodia of macrophages are produced by membrane evagination. Membranes of Organelle Membranes of endoplasmic reticulum, nucleus, lysosomes and outer layer of mitochondria may be considered as variants of plasma membrane. Percentage of protein content varies from 20% in myelin sheath to over 70% in the inner membranes of mitochondria. Cytoskeleton Human body is supported by the skeletal system; similarly the structure of a cell is maintained by the cytoskeleton present underneath the plasma membrane. Water soluble compounds are generally impermeable and require carrier mediated transport. An important function of the membrane is to withhold unwanted molecules, while permitting entry of molecules necessary for cellular metabolism. Acetyl choline receptor 14 Textbook of Biochemistry; Section A: Chemical Basis of Life solute in the hydrophobic core of the membrane. This mechanism does not require energy but the rate of transport is more rapid than simple diffusion process. In the pong state, the active sites are exposed to the exterior, when the solutes bind to the specific sites. In the ping state, the active sites are facing the interior of the cell, where the concentration of the solute is minimal.
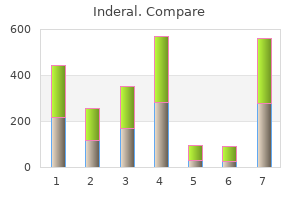
Inderal 80 mg otc. New blood pressure guidelines affect Kansans.
Answer:T the transport of food into cells involves the action of the sodiumpotassium pump and coupled channels blood pressure ranges low discount inderal 10mg amex. Answer:T Characteristic functions of membranes are mediated by specific proteins arrhythmia newborn buy discount inderal 10 mg online, serving as pumps pulse pressure 37 buy inderal 80mg cheap, channels blood pressure qof cheap inderal 10mg amex, receptors 1 5 generic 10 mg inderal mastercard, energy transducers and enzymes blood pressure medication metoprolol inderal 40mg without prescription. Answer:T 112 Chapter 4 Nucleic Acids Nucleic Acid Building Blocks Nucleic acids are composed of nucleotide monomers, which themselves are built from a phosphate group, a sugar, and a nitrogenous base. The bases are of two types, pyrimidines (single ringed) and purines (double ringed). A nucleoside is an N-glycoside formed between a base and a sugar (usually ribose or deoxyribose). The orientation of the helix is usually right handed with the two chains running antiparallel to one another. Proteins and drugs interact with the functional groups on the bases that are exposed in the grooves. Absorption increases with increasing heat breaking the hydrogen bonds that hold the strands together, unstacking and exposing the bases. Renaturation will only occur if the temperature is below the Tm, however if the temperature is too low the rate of renaturation will decrease. The double stranded nucleic acid that forms is a heteroduplex and the extent of heteroduplexes indicates homology between the two nucleic acid sources. Frederick Griffiths about 1928 studied the R & S strains by injecting them into mice. The Griffiths did a strange experiment and got a strange result: · Boiled S + live R injected into mice -> pneumonia -> death. Answer:D Polyacrylamide and agarose gel electrophoresis separate nucleic acids based primarily on their A. Answer: C In the nucleotides found in nucleic acids, the linkage between the bases and the sugar involve: A. N-9 of purines and C-2 of the sugar Answer: A the process of "transcription" in eukaryotes involves the synthesis of: A. Answer: D How many hydrogen bonds are formed between the complementary bases G:C and A:T, respectively? In recent years, new varieties of farm plants and animals have been engineered by manipulating their genetic instructions to produce new characteristics. Mapping of genetic instructions in cells makes it possible to detect, and perhaps correct, defective genes that may lead to poor health. Substances from genetically engineered organisms have increased the cost and side effects of replacing missing body chemicals. Cell regulation allows cells to respond to their environment and control and coordinate cell growth and division. Cell regulation occurs through both changes in the activity of proteins and selective expression of individual genes. Proteins are long, folded molecules, but do not have specific shapes which influence their functions. Offspring resemble their parents because they inherit similar genes that code for the production of proteins that form similar structures and perform similar functions. Oligonucleotide is a polynucleotide consisting of a few nucleotide Answer: residues is a compound consisting of a nitrogenous base linked to a five-carbon sugar (ribose or deoxyribose). Answer: orphan gen~ is a technique in which a cloned gene is mutated in a specific manner. Answer: Denaturation Genes that are related by evolution from a common ancestor are called genes. Answer: homologous is a nucleotide sequence variation in the genomes of two individuals from the same species. Other amino acids are also found in proteins, but most arise by modification from the twenty after they have been incorporated in the protein. All of the standard amino acids are amino acids (except for proline, an imino acid). There are twenty (or so) amino acids, which we will discuss in groups based on their chemical properties. The hydrophobic amino acids are· Aliphatic: glycine (Gly, G), alanine (Ala, A), leucine (Leu, L), isoleucine (lIe, I), valine (Val, V). The polar amino acids are those that are not charged at physiological pH, but which are nevertheless quite polar due to their alcohol or amide groups. The pKa refers to the dissociation of the proton from a positively charged (protonated) amine group. These disulphide bridges (confusingly known as cystine), are responsible for a lot of protein tertiary structures. The charged (acidic/basic) and polar amino acids are often involved in catalysis, forming covalent products with substrates. In addition to the amino acids found in proteins, the cell also contains a number of other aminp acids that are not normally found in peptides. These modified amino acid residues include the phosphorylated alcohol amino acids (Ser, Thr, Tyr), which are phosphorylated by kinase enzymes. Thus, they will share the chemical reactions of these groups familiar from organic chemistry. Many of these reactions are exploited in the laboratory manipulation of amino acids, peptides, and proteins. Biologically the most important reactions are those required for protein formation, particularly the peptide bond. Classification of Amino Acids the protein amino acids are classified according to the chemical nature of their R groups as aliphatic, aromatic, heterocyclic and sulphur containing amino acids. More meaningful classification of amino acids is based on the polarity of the R groups. The polarity of the R groups varies widely from totally non-polar to highly polar. The side chains of alanine, valine, leucine and isoleucine are important in promoting hydrophobic interactions within protein structures. On the other hand, the imino group of proline is held in a rigid conformation and reduces the structural flexibility of the protein. All these amino acids participate in hydrophobic interactions, which is stronger than aliphatic R groups because of stacking one another. Tyrosine and tryptophan are more 145 Fundamentals ofBiochemistnj: A Textbook polar than phenylalanine due to the presence of hydroxyl group in tyrosine and nitrogen in the indole ring of tryptophan. This property is exploited in the characterization and quantification of proteins. This group of amino acids includes serine, threonine, cysteine, methionine, asparagine and glutamine. The hydroxyl group of serine and threonine, the sulphur atom of cysteine and methionine and the amide group of asparagine and glutamine, contribute to the polarity. The R groups of these amino acids are more hydrophilic than the non-polar amino acids. The lysine has a second E-amino group; arginine has a positively charged guanidino group; and histidine has an imidazole group. Essential Amino Acids Most of the prokaryotic and many eukaryotic organisms (plants) are capable of synthesizing all the amino acids present in the protein. But higher animals including man possess this ability only for certain amino acids. The other amino acids, which are needed for normal functioning of the body but cannot be synthesized from metabolic intermediates, are called essential amino acids. These must be obtained from the diet and a deficiency in anyone of the amino acids prevents growth and may even cause death. Methionine, Threonine, Tryptophan, Valine, Isoleucine, Leucine, Phenylalanine and Lysine are the essential amino acids, however, Histidine and Arginine. Properties of Amino Acids Physical · Amino acids are white crystalline substances. The amino acids possessing both positive and negative charges are called zwitterions. Examination of the structure of amino acids reveals that except glycine, all other amino acids possess asymmetric carbon atom at the position. Chemical Properties · Reaction with formaldehyde (Formal titration): An amino acid solution is treated with excess of neutralized formaldehyde solution, the amino group combines with formaldehyde forming dimethylol amino acid which is an amino acid formaldehyde complex Hence the amino group is protected and the proton released is titrated aga1nst alkali. This method is used to find out the amount of total free amino acids in plant samples. When a solution of amino acid is boiled with ninhydrin, the amino acid is oxidatively deaminated to produce ammonia and a ketoacid. The keto acid is decarboxylated to produce an aldehyde with one carbon atom less than the parent amino acid. This ninhydrin reaction is employed in the quantitative determination of amino acids. Proteins and peptides that have free amino group(s) (in the side chain) will also react and give colour with ninhydrin. Decarboxylation: the carboxyl group of amino acids is decarboxylated to yield the corresponding amines. Thus, the vasoconstrictor agent, histamine · 147 Fundamentals of Biochemistry: A Textbook is produced from histidine. Histamine stimulates the flow of gastric juice into the stomach and the dilation and constriction of specific blood vessels. Excess reaction to histamine causes the symptoms of asthma and various allergic reactions. The covalent bond is formed between the a-carboxyl group of one amino acid and the aamino group of the next amino acid. The bond so formed between the carboxyl and the amino groups, after elimination of a water molecule is called as a peptide bond and the compound formed is a peptide. Thus they will exhibit titration curves similar to a free amino acid, but with the pKa values shifted closer to simple acid and amine values (there will be no charge stabilization). By convention the amino terminal residue is written on the left progressing to the carboxyl terminal residue on the right: +H3N-aa-aa-aaaa-C02-. Can determine the composition of a peptide by acid hydrolysis and amino acid analysis. These proteins are further classified based on their solubility in different solvents as well as their heat coagulability. Albumins Albumins are readily soluble in water, dilute acids and alkalies and coagulated by heat. Globulins Globulins are insoluble or sparingly soluble in water, but their solubility is greatly increased by the addition of neutral salts such as sodium chloride. Glutelins Glutelins are insoluble in water and absolute alcohol but soluble in dilute alkalies and acids. Histones Histones are small and stable basic proteins and contain fairly large amounts of basic amino acid, histidine. Protamines are found in association with nucleic acid in the sperm cells of certain fish. These are called albuminoids because they are essentially similar to albumin and globulins. They occur as chief constituent of exoskeleton structure such as hair, horn and nails. The nature of the non-protein or prosthetic groups is the basis for the sub classification of conjugated proteins. Nucleoproteins Nucleoproteins are simple basic proteins (protamines or his tones) in salt combination with nucleic acids as the prosthetic group. Mucoproteins these proteins are composed of simple proteins in combination with carbohydrates like mucopolysaccharides, which include hyaluronic acid and chondroitin sulphates. On hydrolysis, mucopolysaccharides yield more than 4 % of amino-sugars, hexosamine and uronic acid. Soluble mucoproteins are neither readily denatured by heat nor easily precipitated by common protein precipitants like trichloroacetic acid or picric acid. The term glycoproteins is restricted to those proteins that contain small amounts of carbohydrate usually less than 4 % hexosamine. Lipoproteins these are proteins conjugated with lipids such as neutral fat, phospholipids and cholesterol. A f3-globulin, termed transferrin is capable of combining with iron, copper and zinc. Phosphoric acid is linked to the hydroxyl group of certain amino acids like serine in the protein, Example- casein of milk. They include two types of derivatives, primary-derived proteins and secondary-derived proteins. Primary-derived Proteins these protein derivatives are formed by processes causing only slight changes in the protein molecule and its properties. Proteans Proteans are insoluble products formed by the action of water, dilute acids and enzymes. These are particularly formed from globulins but are insoluble in dilute salt solutions. Coagulated Proteins Coagulated proteins are insoluble products formed by the action of heat or alcohol on natural proteins. Secondary-derived Proteins these proteins are formed in the progressive hydrolytic cleavage of the peptide bonds of protein molecule. They are roughly grouped into proteoses, pep tones and peptides according to average molecular weight. Proteoses are hydrolytic products of proteins, which are soluble in water and are not coagulated by heat.
It is what you do when you walk down to the store blood pressure medication parkinson's order inderal 10mg without a prescription, fidget heart attack indigestion buy 40 mg inderal, garden blood pressure medication side effects cough order 80 mg inderal visa, clean the house blood pressure medication addiction order 40 mg inderal, have sex or type on your computer exforge blood pressure medication buy 40mg inderal overnight delivery. Research looking at a person who sits all day long and then does a 30-minute workout (metabolics) compared to a person who moves all day long but does not do structured exercise suggests that the mover is far better off health and fitness wise than the metabolic exerciser pulse pressure ati buy inderal 40 mg cheap. This is one of the reasons why policy makers suggest walking 10,000 steps per day. It is a way to make sure that we move more in the way that our naturally thin ancestors did. And, surprisingly, these people were much leaner, fitter and healthier than we are today. By all accounts, more people do structured exercise today than at any other time in the history of mankind, yet the modern day exercising man or woman is heavier than past generations. At first this discrepancy may seem to make little sense until you look at the statistics on moving. Research shows that our hunter-gatherer ancestors walked from 7 to 20 miles every single day, often carrying babies and hauling gear in the process. In one recent study published in the May 2013 issue of the journal Diabetologia,36 researchers showed that movement was a far better predictor of health than either moderate or even intense physical exercise. In other words, sitting all day long and then going for a vigorous 30-minute run was not nearly as effective for health and metabolic function as just moving more. This research, and other studies like it, have led many experts in the health and fitness fields to begin focusing much of their efforts on getting people to move more rather than exercise more. Some of the new recommendations coming out of this research hints that organizations such as the American Heart Association, the American Diabetes Association and the American College of Sports Medicine should set a daily limit on sitting time. I realize this information can be a little confusing, but once you think about it, it starts to make intuitive sense. But can simply moving more really help with weight loss and be better than exercise? A study in the October 2005 issue of the journal Chest showed that jogging for 12 miles a week was not much different than walking for 12 miles a week from a weight loss perspective and that both dramatically enhanced cardiovascular health. Intense exercise releases more cortisol into the body, and this can have damaging effects. The take home is that more exercise is not really that beneficial, but more movement is. You would be better off moving all day than sitting all day and then doing an intense bout of exercise. Keep in mind that 5,000 steps is a little less than an hour a day for most people. Normal Menstrual Cycle-Estrogen and Progesterone Balanced Given your more balanced and stable hormonal rhythms, you should be able to benefit from as much walking as you like. Normal Menstrual Cycle-Estrogen Dominant Given the extra estrogen you may be dealing with, you will want to shoot for the upper limit of walking. For you 10,000 steps a day should be a minimum, while 20,000 steps per day is probably the maximum you need. Normal Menstrual Cycle-Progesterone Deficient Because you are dealing with stress issues-one of the major causes of progesterone falling-you will want to make sure walking is done enough but not too much. Then every few weeks you can increase the walking load by 1,000 to 2,000 steps per day. Remember, both estrogen and progesterone work together to keep a lid on the negative effects of cortisol. This is one of the major causes of being normal weight but still dealing with belly fat issues. You will want to use walking very carefully since for you it can be more easily overdone. Start with 5,000 steps per day and then slowly increase that by 5,000 steps per day each week. You also may want to consider walking while listening to slow relaxing spa music to make sure you go slow and take your time. Perimenopause-Estrogen Fluctuating, Progesterone Deficient this is a volatile time where some days you will feel balanced and productive and other days you may feel crazy. Walking a little faster and more briskly (somewhere between a slow walk and powerwalking, i. Reducing the speed at night to a slow meander while practicing deep breathing along with the walking can help with sleep and anxiety. Menopause-Estrogen and Progesterone Deficient Now that you have made it through perimenopause, things are a lot less volatile. You can and should definitely ramp up your walking to between 10,000 and 20,000 steps per day, which means 2 to 4 hours daily. I realize this seems like a lot, but this can be accumulated in all daily activity. To make your movement work best for you try to do two to three 30 to 40 minute walks throughout the day in addition to staying active and on your feet. Walking is hard for you to overdo and is one of the few things where more is almost always better. Post-Menopause-Estrogen and Progesterone Deficient At this stage of life you are still dealing with low estrogen and progesterone, but also a higher relative level of testosterone. Walking is the belly fat killer, because it helps buffer against the insulin and cortisol effects induced by the loss of female hormones. You can do this through 2 two hour walks, four 1 hour walks, or just accumulate the steps through your general daily activities. In fact, there may be a synergistic effect when walking is combined with intense exercise. While elevated cortisol is good and beneficial for fat-burning during exercise, it is not great for it to remain high after exercise. Doing your walk after your workout also gets you up and moving more without an exorbitant time investment. Walk to the market, take the stairs at work, go out for a gentle hike with your family, or do whatever else gets you up and moving more! Moving all day and not exercising may be better than sitting still all day and then exercising. Walking mixed with intelligent metabolic-enhancing exercise may be the most powerful combination of all. While elevated cortisol is good and beneficial for fat burning during exercise it is not great to have high cortisol after exercise. Add the tips I outlined for how to further optimize the meal plan based on your hormone type. Follow the delicious menus and recipes in the 12-Week Metabolic Renewal Meal Plan. We have included specific guidelines on what foods to eat, what foods not to eat, added some suggestions for snacks and additional meals if that applies to you, and more. The recipes we have developed are not only spectacularly delicious, but we have kept them to the absolute highest nutritional standards we could. A new set of moves wake up your 100 cells and your mitochondria (your cellular energy factories) to turn fat into energy at top speed. One final consideration on the exercises is to make sure you pay close attention to my cues during the workouts. For example, to get the most out of the squeezing exercises, you will need to stay focused. Do your best not to rush the exercises and really work on squeezing tight on the areas you are working. The Burnout Session I have also included a little something extra in the Metabolic Renewal workouts. The burnout session is a super-intense callisthenic and plyometric workout that is designed to accentuate the breathless component of the workout. It creates an even greater metabolic effect, but it also makes doubly sure that we can burn up all the fat that was released during the workout. You goal is to push yourself as hard as you can within your fitness level until you are forced to stop and rest. The 15 minutes is truly 15 minutes of elapsed time, which includes both your exercise and your rest time. The most beneficial workouts will have you resting almost as frequently as you are working. Some people will naturally gravitate to longer rests that are less frequent and others will prefer shorter, more frequent rest periods. Without rest, the workout quality will be compromised and you will revert back to a less efficient style of training. The burnout session is extra insurance that your muscles can mop up all the fat that was mobilized from the main workout. The best way to make sure this fat is burned and not restored is to put a little more demand on your metabolism at the end. You need to convince your body that it not only needs to burn the fat that is released, but do it quickly. It is there for those who feel like they have a little extra in them, and love and want a different challenge. Depending on where you are in your cycle or phase of life, these burnout sessions can either be just right or a little too much. Finishing with a Walk and Cool Down In reality, the best way to ensure that the workout has maximum benefit is to include a leisurely walk after the workout, if you have time. While we want cortisol and catecholamines high during the workout, we want to shut them down when the workout is over. I did not include a standard cooldown in the Metabolic Renewal workouts because I have found a lot of people do their own thing anyway. Your lifestyle should be constantly programming your body to handle stress better. There are so many different types of activities that optimize the metabolic process through reprogramming stress reactions. I call these rest and recovery activities and you should be shooting to include at least one of these activities per day. I have provided specific recommendations for each hormone type as well as a bunch of others you can try if you want. These activities include creative pursuits like writing, painting or other artistic activities. Sitting quietly with herbal tea looking out the window watching the birds, listening to relaxing music, sitting quietly on a park bench watching the world go by, or reading a book, are all forms of mindfulness and meditation. Orgasm, whether through masturbation or sex, is one of the more enjoyable ways to maximize results in this program. In fact, women compared to men have unique brain changes after orgasm that result in more focus, greater pain tolerance, and lower levels of stress hormones. It may be that due to changes in focus and pain reduction, that orgasms both before exercise and after may have benefits for women, where only after may benefit men. Use all these activities to your advantage by scheduling, prioritizing and emphasizing them in your life. Exercise is often not enough to overcome a lifestyle that involves sitting all day every day. Investing in an activity tracker and seeking to accumulate 30,000 to 70,000 steps a week is a great start. Well, there are several reasons and I explain them all in your Metabolic Renewal Transformation Tracker. We humans are capable of incredible delusion, and the only way to get around that is to have objective data and feedback. Tracking takes psychology out of the equation and allows us to make choices about our diet and exercise habits based on objective data. Then each week over the course of the program you will track your results to see how much progress you have made. The Metabolic Renewal program is a 12-week, 4-phase system that contains 3 different workouts per week. After each workout you can complete the burnout session if you want to burn up a little more fat. My favorite cooldown is a leisurely walk, but you can also cool down with full-body stretching. Lifestyle changes-especially walking, relaxation, and detoxification-are extremely important as well. Tracking your results is one of the most important things you can do, as it takes psychology out of the equation, providing you with objective feedback and data. In this section, you will learn how to both amplify and speed your fat loss results. It does not care about your vanity concerns, your timetables, or whether things are convenient for you or not. The metabolism is an adaptive-reactive system that thrives in an environment where food intake and exercise output are more closely aligned. Add to this the cyclical approach to dieting you have now learned, and you can greatly amplify your results. The reason they backfire on almost everyone is due to one simple fact: That is all people know and so that is all they do. In some people, these protocols never result in metabolic compensation and thus can be followed for life.