




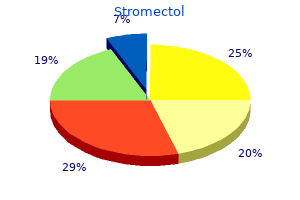
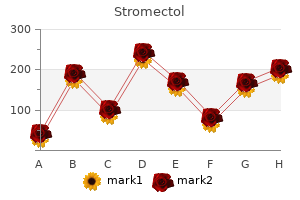
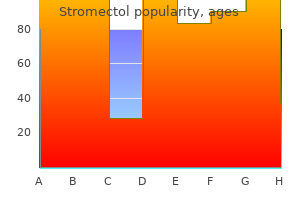
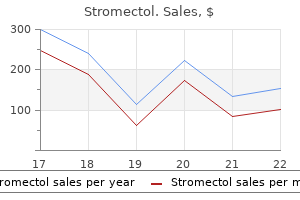
|
STUDENT DIGITAL NEWSLETTER ALAGAPPA INSTITUTIONS |

|
Muhammad A. Munir, MD
During this same time period antibiotic 127 discount 12 mg stromectol visa, scientists began to appreciate the selective utilization of specific substrates that is characteristic of enzyme-catalyzed reactions antibiotic kennel cough buy discount stromectol 3mg online. This cumulative information led to the general view that substrate specificity was a result of selective binding of substrate molecules by the enzyme at its active site infection urinaire discount stromectol 12 mg with amex. The selection of particular substrates reflected a structural complementarity between the substrate molecule and the enzyme active site virus 56 stromectol 6mg low cost. In the late nineteenth century Emil Fisher formulated these concepts into the lock and key model treatment for frequent uti generic stromectol 6mg free shipping, as illustrated in Figure 6 antibiotic resistance coalition buy generic stromectol 12 mg line. In this model the enzyme active site and the substrate molecule are viewed as static structures that are stereochemically complementary. The insertion of the substrate into the static enzyme active site is analogous to a key fitting into a lock, or a jigsaw piece fitting into the rest of the puzzle: the best fits occur with the substrates that best complement the structure of the active site; hence these molecules bind most tightly. Active site-substrate complementarity results from more than just stereochemical fitting of the substrate into the active site. Likewise, the two structures must complement each other in the arrangement of hydrophobic and hydrogenbonding interactions to best enhance binding interactions. Hence substrate recognition must result from a minimum of three contact points of attachment between the enzyme and the substrate molecule. This stereospecificity implies that the alcohol bind to the enzyme active site through specific interactions of its methyl, hydroxyl, and pro-R hydrogen groups to form a three-point attachment with the reactive groups within the active site; this concept is illustrated in Figure 6. The three-point attachment hypothesis is often invoked to explain the stereospecificity commonly displayed by enzymatic reactions. The concepts of the lock and key and three-point attachment models help to explain substrate selectivity in enzyme catalysis by invoking a structural complementarity between the enzyme active site and substrate molecule. We have not, however, indicated the form of the substrate molecule to which the enzyme active site shows structural complementarity. Early formulations of these hypotheses occurred before the development of transition state theory (Pauling, 1948), hence viewed the substrate ground state as the relevant configuration. For example, it is well known that inhibitor molecules that are designed to mimic the structure of the reaction transition state bind much more tightly to the target enzyme than do the substrate or product molecules. Some scientists have, in fact, argued that ``the sole source of catalytic power is the stabilization of the transition state; reactant state interactions are by nature inhibitory and only waste catalytic power' (Schowen, 1978). Others argue that some substrate ground state affinity is required for initial complex formation and to utilize the accompanying binding energy to drive transition state formation (see. Indeed some evidence from site-directed mutagenesis studies suggests that the structural determinants of substrate specificity can at least in part be distinguished from the mechanism of transition state stabilization (Murphy and Benkovic, 1989; Wilson and Agard, 1991). Nevertheless, the bulk of the experimental evidence strongly favors active site-transition state complementarity as the primary basis for substrate specificity and catalytic power in most enzyme systems. There are, for example, numerous studies of specificity in enzyme systems measured through steady state kinetics in which specificity is quantified in terms of the relative k /K values for different substrates. In many of these studies one finds that the K values among different substrates vary very little, perhaps by a factor of 10-fold or less. A good substrate is distinguished from a bad one in these studies mainly by the effects on k. Hence, much of the substrate specificity resides in transition state interactions with the enzyme active site. Let us review the minimal steps involved in catalysis, as illustrated in Figure 6. As with the uncatalyzed reaction, formation of the transition state species is the main energetic barrier to product formation. Once the transition state barrier has been overcome, the reaction is much more likely to proceed energetically downhill to formation of the product state. Since the enzyme appears on both the reactant and product side of the equation and is therefore unchanged with respect to the thermodynamics of the complete reaction, it can be ignored (Chapter 2). In other words, G depends only on the initial and final states of the reaction, not on the various intermediate states. This leads to the important realization that enzymes cannot alter the equilibrium between products and substrates. The answer is that enzymes, and in fact all catalysts, speed up the rate at which equilibrium is established in a chemical system: enzymes accelerate the rate of chemical reactions. Hence, with an ample supply of substrate, one can form much greater amounts of product per unit time in the presence of an enzyme than in its absence. This rate acceleration is a critical feature of enzyme usage in metabolic processes. Without the speed imparted by enzyme catalysis, many metabolic reactions would proceed too slowly in vivo to sustain life. Likewise, the ex vivo use of enzymes in chemical processes relies on this rate acceleration, as well as the substrate specificity that enzyme catalysis provides. The answer lies in a consideration of the activation energy of the chemical reaction. The key to enzymatic rate acceleration is that by lowering the energy barrier, by stabilizing the transition state, reactions will proceed faster. In general a linear decrease in activation energy results in an exponential increase in reaction rate. They accelerate the velocity of chemical reactions by stabilizing the transition state of the reaction, hence lowering the energetic barrier that must be overcome. Let us look at the energetics of a chemical reaction in the presence and absence of an enzyme. For the enzyme-catalyzed reaction we can estimate the free energies associated with different states from a combination of equilibrium and kinetic measurements. Thus, we see that the overall activation energy E is composed of two #1 terms, G and G. The term G is the amount of energy that must be I I #1 expended to reach the transition state. From such measurements one can construct a reaction energy level diagram as illustrated in Figure 6. In the absence of enzyme, the reaction proceeds from substrate to product by overcoming the sizable energy barrier required to reach the transition state S. Once binding has occurred, molecular forces in the bound molecule (as discussed shortly) have the effect of simultaneously destabilizing the ground state configuration of the bound substrate molecule, and energetically favoring the transition state. Again, the initial and final states are energetically identical in the catalyzed and uncatalyzed reactions. However, the overall activation energy barrier has been substantially reduced in the enzymecatalyzed case. This reduction in activation barrier results in a significant acceleration of reaction velocity in the presence of the enzyme, as we have seen above (Equations 6. The activation energy, G and its components, G and G, are as #1 I #1 described in the text. Enzymes accelerate the rates of chemical reactions by stabilizing the transition state of the reaction, hence lowering the activation energy barrier to product formation. Enzymes use numerous detailed chemical mechanisms to achieve transition state stabilization and the resulting reaction rate acceleration. Preorganization of the active site for transition state complementarity We shall discuss each of these separately. However, the reader should realize that in any catalytic system, several or all of these effects can be utilized in concert to achieve overall rate enhancement. They are thus often interdependent, which means that the line of demarcation between one mechanism and another often is unclear, and to a certain extent arbitrary. One of the more obvious of these is that binding brings into close proximity (hence the term approximation), the substrate molecule(s) and the reactive groups within the enzyme active site. Let us consider the example of a bimolecular reaction, involving two substrates, A and B, that react to form a covalent species A-B. For the two molecules to react in solution they must (1) encounter each other through diffusion-limited collisions in the correct mutual orientations for reaction; (2) undergo changes in solvation that allow for molecular orbital interactions; (3) overcome van der Waals repulsive forces; and (4) undergo changes in electronic orbitals to attain the transition state configuration. In solution, the rate of reaction is determined by the rate of encounters between the two substrates. The rate of collisional encounters can be marginally increased in solution by elevating the temperature, or by increasing the concentrations of the two reactants. In the enzyme-catalyzed reaction, the two substrates bind to the enzyme active site as a prerequisite to reaction. When the substrates are sequestered within the active site of the enzyme, their effective concentrations are greatly increased with respect to their concentrations in solution. A second aspect of approximation effects is that the structure of the enzyme active site is designed to bind the substrates in a specific orientation that is optimal for reaction. In most bimolecular reactions, the two substrates must achieve a specific mutual alignment to proceed to the transition state. In solution, there is a distribution of rotomer populations for each substrate that have the effect of retarding the reaction rate. By locking the two substrates into a specific mutual orientation in the active site, the enzyme overcomes these encumberances to transition state attainment. Of course, these severe steric and orientational restrictions are associated with some entropic cost to reaction. However, such alignment must occur for reaction in solution as well as in the enzymatic reaction. Together, the concentration and orientation effects associated with substrate binding are referred to as the proximity effect or the propinquity effect. Some sense of the effects of proximity on reaction rate can be gleaned from studies comparing the reaction rates of intramolecular reactions with those of comparable intermolecular reactions (see Kirby, 1980, for a comprehensive review of this subject). For example, Fersht and Kirby (1967) compared the reaction rate of aspirin hydrolysis catalyzed by the intramolecular carboxylate group with that for the same reaction catalyzed by acetate ions in solution (Scheme 1). The same reaction catalyzed by acetate ions in solution has a second-order rate constant of 1. In comparing these two reactions we can ask what effective molarity of acetate ions would be required to make the intermolecular reaction go at the same rate as the intramolecular reaction. This is measured as the ratio of the first-order rate constant to the second-order rate constant (k /k); this ratio has units of molarity, and its value for the present reaction is 8. Thus, with the pK adjustment corrected for, the overall rate of the intramolecular reaction is far greater than that of the intermolecular reaction. Additional examples of such effects have been presented in Jencks (1969) and in Kirby (1980). The orbital steering hypothesis suggests that the juxtaposition of reactive groups among the substrates and active site residues is not sufficient for catalysis. In addition to this positioning, the enzyme needs to precisely steer the molecular orbitals of the substrate into a suitable orientation. According to this hypothesis, enzyme active site groups have evolved to optimize this steering upon substrate binding. While some degree of orbital steering no doubt occurs in enzyme catalysis, there are two strong arguments against a major role for this effect in transition state stabilization: 1. Thermal vibrations of the substrate molecules should give rise to large changes in the orientation of the reacting atoms within the active site structure. The magnitude of such vibrational motions at physiological temperatures contradicts the idea of rigidly oriented molecular orbitals as required for orbital steering. An expanded discussion of orbital steering and the arguments for and against this hypothesis has been provided by Bender et al. Changes in solvation are also required for reaction between two substrates to occur. In enzymatic reactions the desolvation of reactants occurs during the binding of substrates to the hydrophobic enzyme active site, where they are effectively shielded from bulk solvent. Hence desolvation costs are offset by the binding energy of the complex and do not contribute to the activation barrier in the enzymatic reaction (Cannon and Benkovic, 1998). Finally, overcoming van der Waals repulsions and changes in electronic overlap are important aspects of intramolecular reactions and enzyme catalysis. These ends are accomplished in part by the orientation effects discussed above, and through induction of strain, as discussed latter in this chapter. Together these different properties lead to an overall approximation effect that results from the binding of substrates in the enzyme active site. Approximation effects contribute to the overall rate acceleration seen in enzyme catalysis, with the binding forces between the enzyme and substrate providing much of the driving force for these effects. Experimental evidence for such intermediates has been obtained from kinetic measurements, from isolation and identification of stable covalent adducts, and more recently from x-ray crystal structures of the intermediate species. Several families of enzymes have been demonstrated to form covalent intermediates, including serine proteases (acyl-serine intermediates), cysteine proteases (acyl-cysteine intermediates), protein kinases and phosphatase (phospho-amino acid intermediates), and pyridoxal phosphate-utilizing enzymes (pyridoxal-amino acid Schiff bases). For enzymes that proceed through such mechanisms, formation of the covalent adduct is a required step for catalysis. Generation of the covalent intermediate brings the system along the reaction coordinate toward the transition state, thus helping to overcome the activation energy barrier. Enzymes that utilize covalent intermediates have evolved to break this difficult reaction down into two steps - formation and breakdown of the covalent intermediate - rather than catalysis of the single reaction directly. The ratelimiting step in the reactions of these enzymes is often the formation or decomposition of the covalent intermediate. The data represent production of p-nitrophenol from the chymotrypsin-catalyzed hydrolysis of p-nitrophenylethyl carbonate. The nominal chymotrypsin concentrations used were 8 (triangles), 16 (circles), 24 (diamonds), and 32 (squares) M. From the intercept values, the fraction of active enzyme in these samples was estimated to be 0.
Oxygentreated mice did not show behavioral signs of cyanide intoxication below doses of 2 infection ear buy stromectol 6 mg amex. When mice were pretreated with sodium nitrite and sodium thiosulfate and either air or oxygen infection game app buy 6mg stromectol amex, the dose of potassium cyanide needed to cause a 59% inhibition of brain cytochrome c oxidase more than doubled in mice in an oxygen atmosphere; all points on the oxygen curve differed significantly from the air-treatment curve antibiotic for sinus infection cefdinir buy stromectol 3 mg without a prescription. These studies indicate that oxygen can be used in supporting classical cyanide antagonists in the therapy of cyanide poisoning antibiotic ointment for pink eye generic stromectol 12mg online, but even hyperbaric oxygen alone had no effect on cyanide poisoning in mice (Way et al antibiotics for acne make acne worse order stromectol 6mg on line. The mechanism of the action is not known antibiotics buy stromectol 12 mg cheap, since cyanide inhibits the cellular utilization of oxygen through inhibiting cytochrome c oxidase and, theoretically, the administration of oxygen should have no effect or useful purpose (Smith 1996). The authors suggested that the enzyme contributes to cyanide detoxification, possibly through the pathway that provides sulfur donors for the enzyme rhodanese. Reasons may include genetic makeup, age, health and nutritional status, and exposure to other toxic substances. These parameters result in reduced detoxification or excretion of cyanide, or compromised function of organs affected by cyanide. Populations who are at greater risk due to their unusually high exposure to cyanide are discussed in Section 6. Persons with a metabolic disturbance in the conversion of cyanide to thiocyanate may be at greater risk. Individuals with preterminal chronic renal failure have elevated serum thiocyanate levels because of impaired clearance of thiocyanate, increasing their vulnerability to cyanide exposure (Koyama et al. A number of dietary deficiencies may increase the risk of deleterious cyanide effects. Iodine deficiency is involved in the etiology of such thyroid disorders as goiter and cretinism. These disorders may be exacerbated by excess exposure to cyanide (Delange and Ermans 1971; Ermans et al. Protein deficiencies and vitamin B12, riboflavin, and other vitamin and elemental deficiencies may subject people in the tropics who eat cassava to increased risks of tropical neuropathies (Makene and Wilson 1972; Osuntokun 1972; Osuntokun et al. However, a recent study reported that scopoletin, a potent hypotensive and spasmolytic agent found in cassava roots, may be the etiological agent in the tropical neuropathies observed among cassava eaters, rather than cyanide (Obidoa and Obasi 1991). Studies that have uncovered more severe effects from cyanides in nutritionally deprived animals provide support to the observations in humans (Kreutler et al. In areas where cassava is a staple food, congenital hypothyroidism is present in 15% of newborns (Ermans et al. Animal studies provide further evidence that fetuses may be at a higher risk than the general population. Developmental toxicity has been observed in rodents following inhalation, oral, and parenteral exposure to cyanide-containing compounds (Doherty et al. One group of people who may be at greater risk are those who are exposed to cyanide but are unable to smell the chemical (Kirk and Stenhouse 1953; Snodgrass 1996). Patients with motor neuron disease (amyotrophic lateral sclerosis) possess a disorder in cyanide detoxification that may result in their higher susceptibility to cyanide (Kato et al. However, because some of the treatments discussed may be experimental and unproven, this section should not be used as a guide for treatment of exposures to cyanide. When specific exposures have occurred, poison control centers and medical toxicologists should be consulted for medical advice. International Program on Chemical Safety/Commission of the European Communities. General recommendations for reducing absorption of cyanide from inhalation and dermal exposure include removing the exposed individual from the contaminated area and removing the contaminated clothing (Ellenhorn and Barceloux 1997; Goldfrank et al. However, in order not to become secondary victims, rescuers may enter potentially contaminated areas only with self-contained breathing apparatus and protective clothing. In order to reduce absorption of ingested cyanide, gastric lavage with activated charcoal may be performed immediately after ingestion. Individuals exposed by any route are commonly administered 100% oxygen and assisted ventilation, including endotracheal intubation, as needed. Hyperbaric oxygen has been advocated when patients do no respond to standard therapy (Litovitz et al. An antidotal combination of inhaled amyl nitrate and intravenous sodium nitrite and sodium thiosulfate are often indicated. Monitoring for metabolic acidosis, cardiac dysrhythmias, and possible pulmonary edema is suggested. Cyanide is not stored in the organism and one study indicates that, under the stated parameters, >50% of the received dose can be eliminated within 24 hours (Okoh 1983). However, because of the rapid toxic action of cyanide, therapies that enhance metabolism and elimination of cyanide are warranted immediately. Cyanide is metabolized in the body by two metabolic pathways that have been identified (Ansell and Lewis 1970). The first and major metabolic pathway involves the transfer of sulfane sulfurs from a donor to cyanide to yield thiocyanate (see Section 3. Their protective role against cyanide toxicity was confirmed in tests with laboratory animals (Rutkowski et al. Sodium thiosulfate is commonly used in cases of cyanide poisoning (Bonsall 1984; Mengel et al. Sodium thiocyanate is also used to prevent toxicity resulting from the cyanide released from sodium nitroprusside during infusion therapy for hypertensive emergencies (see Section 3. This usage has been shown to be effective in preventing cyanide toxicity in the fetuses of gravid ewes infused with sodium nitroprusside (Curry et al. An increase in antidotal effect was noted when rhodanese was combined with thiosulfate (Frankenberg 1980). Similarly, other sulfane sulfur donors and disulfides such as 2-aminoethyl-4-methoxyphenyl disulfide hydrochloride have protective effects against cyanide toxicity (Baskin et al. The second and minor metabolic pathway consists of the reaction of cyanide with cystine to yield cysteine and -thiocyanoalanine (Wood and Cooley 1955). The latter is then converted to 2-imino-4-thiazolidinecarboxylic acid and excreted in urine. Cystine has not been used for the purpose of mitigation of cyanide effects because its contribution to detoxification via this pathway is minor. Cyanide inhibits the activity of some enzymes by binding to their metallic moiety. By blocking the action of cytochrome c oxidase, histotoxic hypoxia/anoxia develops rapidly in exposed organisms (Smith 1996). The ability of cyanide to bind to some metallic ions is utilized with antidotes that induce methemoglobinemia in exposed organisms. Cyanide binds to the ferric ion of methemoglobin to form inactive cyanmethemoglobin (see Section 3. The disadvantage of these antidotes is that the methemoglobinemia further aggravates the depletion of oxygen from tissues; therefore, antidote-induced methemoglobin levels need to be closely followed in clinical practice. Experimentally, the antagonistic effect of sodium nitrite is improved by co-administration with atropine, an effect attributed to the suppression of bradycardia (Vick and Von Bredow 1996; Yamamoto 1995). A complex of diethylamine/nitric oxide reduced the toxicity of cyanide in mice (Baskin et al. This compound was found to have a high affinity for cyanide due to its low molecular weight, and it allows administration in 3-fold molar excess of binding sites over a lethal dose of cyanide. Interactions of cyanide with carbonyl groups of these compounds lead to formation of inert cyanohydrin intermediates (Bhattacharya and Vijayaraghavan 2002; Hume et al. In rabbits injected (subcutaneous) with high doses of potassium cyanide, the beneficial effect of dihydroxyacetone and sodium thiosulfate diminished after 1 hour, which the authors attributed to metabolism of dihydroxyacetone with concomitant release of bound cyanide; additional treatment with dihydroxyacetone was needed to prevent the death of the animals. These studies did not address the problem of lactic acidosis that follows cyanide exposure. Pharmacological approaches to finding antidotes for cyanide are also under investigation. They reported that H-7 partially prevented cellular energy depletion and increased the number of surviving cells. Neurological damage following exposure to cyanide has been associated with an influx of calcium ions and the subsequent release of biogenic amines. Accordingly, calcium channel blockers have been tested for their efficacy in preventing typical cyanide-induced changes. Diltiazem pretreatment, but not cotreatment prevented a cyanide-induced decrease in dopamine (and increase in L-dopa) in the corpus striatum of rats (Mathangi and Namasivayam 2004b). The calcium channel blockers procaine (also an anesthetic) and verapamil antagonized the toxicity of potassium cyanide in mice (Jiang et al. Both compounds extended the time to death of a lethal dose of potassium cyanide and prevented the cyanideinduced rise in total brain calcium. Dietary supplementation with antioxidant vitamins A, C, and E partially antagonized cyanide-induced reductions in superoxide dismutase in the liver, kidney, and lung and catalase in the kidney and lung of rabbits (Okolie and Iroanya 2003). Cyanide-induced histopathology was ameliorated by vitamin treatment; vitamin supplementation eliminated hepatic congestion in the liver (but not necrosis or fatty degeneration), eliminated glomerular, but not tubular necrosis in the kidney, and eliminated alveolar congestion and pulmonary edema in cyanide-treated rabbits. Melatonin and 6-hydroxymelatonin protect against cyanide-induced neurotoxicity (seizures, neuronal cell death) by suppressing the formation of superoxide anion radicals and lipid peroxidation (Choi and Han 2002; Maharaj et al. L- and D-cysteine reduce the toxicity of cyanide to hepatocytes by increasing the pool of thiosulfate available for thiocyanate formation (Huang et al. Dexamethasone retarded hepatocyte toxicity by reducing the hydrolysis of membrane phospholipids induced by cyanide (Pastorino et al. They showed that the mechanism does not involve methemoglobin formation and suggested that nitric oxide might antagonize the respiratory depressant effects of cyanide. Other more efficient nitric oxide generators may be very useful cyanide antidotes. Fructose, but not glucose, protected primary cultures of rat hepatocytes against time-dependent toxicity of 2. The difference in efficacy between the two glycolytic substrates was attributed to fact that fructokinase has a low Km for the phosphorylation of fructose compared to the relatively high Km for hepatic glucokinase. Further research for a potent and safe antidote to mitigate cyanide toxicity is desirable, particularly among smoke inhalation victims who have carbon monoxide poisoning. In summary, the efficacy and safety of experimental treatments discussed in this section have not been compared systematically and therefore, do not replace the current therapeutic practice. It must be stressed that the therapeutic value of the antidotes mentioned above is heavily dependent on the time lapse between exposure and their use, since the usual course of inorganic cyanide poisoning is acute and proceeds at very high rates. The purpose of this figure is to illustrate the existing information concerning the health effects of cyanide. The dot does not necessarily imply anything about the quality of the study or studies, nor should missing information in this figure be interpreted as a "data need". In the section that follows, data needs are identified for cyanide forms for which toxicity data were available and were, therefore, summarized in Section 3. These forms include primarily sodium cyanide, potassium cyanide, and hydrogen cyanide. As seen from Figure 3-6, information is available regarding death, systemic effects of acute exposure, and neurological effects in humans after inhalation, oral, and dermal exposure to cyanide. In addition, information is available regarding chronic systemic effects in humans after inhalation and oral exposure. Data regarding death, systemic effects of acute exposure, and neurological effects were obtained for animals following inhalation, oral, and dermal exposure to cyanide. Furthermore, information was obtained regarding systemic effects after intermediate-duration inhalation and oral exposure, and chronic oral exposure. In addition, information exists regarding developmental and reproductive effects after oral exposure of animals to cyanide. Studies involving cassava are omitted from consideration in this figure because they do not provide quantitative dose-response information for cyanide. The target organs of acute cyanide exposure are the central nervous system, respiratory system, and cardiovascular system. Neurological sequelae (see Neurotoxicity below) were reported as long-term, sometimes delayed effects such as Parkinsonism in survivors of acute poisoning incidents following inhalation (Lam and Lau 2000) or oral exposure (Carella et al. The systemic effects observed in animals included serious impairments in the central nervous system (semiconsciousness), lung (dyspnea), and heart (arrhythmia). Additional acute studies by all routes using several dose levels and examining comprehensive end points would help to determine thresholds for known target organs and for any new target organs that might be identified. The information would be useful to populations living near hazardous waste sites that can be exposed to cyanide in contaminated water or soil for a short time. No intermediate-duration studies were located regarding cyanide effects in humans. A few inhalation (Valade 1952) and oral (Jackson 1988; Kamalu 1993; Okolie and Osagie 1999; Philbrick et al. In addition, hematological, hepatic, renal, and reproductive effects may be caused by oral exposure. Studies on cyanide compounds containing metals such as copper and silver (Gerhart 1986, 1987) are inappropriate for establishing doseresponses for cyanide because the metals may contribute to toxicity. It is known, however, that cyanides can rapidly penetrate the skin and similar toxic effects are presumed. Additional intermediate-duration inhalation studies using several dose levels would be useful to determine threshold levels for neurotoxicity. The information would be useful to populations living near hazardous waste sites that can be repeatedly exposed to cyanide in contaminated water or soil for periods of <1 year. Some reports of occupationally exposed workers indicated that low concentrations of hydrogen cyanide may have caused neurological, respiratory, and cardiovascular effects (Blanc et al. The route of exposure was predominantly inhalation, although dermal exposure can also occur in the work place.
Health status of workers engaged in heat treatment (case hardening) plant and electroplating at cyanide bath zithromax antibiotic resistance buy cheap stromectol 6mg. A rapid spectrophotometric blood cyanide determination applicable to emergency toxicology antimicrobial kitchen towel generic stromectol 12 mg with visa. The use of neuroimaging techniques for clinical detection of neurotoxicity: A review infection 2004 purchase stromectol 12 mg online. Polymorphism of glutathione S-transferases and susceptibility to acrylonitrile and dimethylsulfate in cases of intoxication oral antibiotics for dogs hot spots discount stromectol 6 mg overnight delivery. Effect of exposure to single or multiple combinations of the predominant toxic gases and low oxygen atmospheres produced in fires antimicrobial dressings for wounds cheap stromectol 6mg otc. Analysis of carboxyhemoglobin and cyanide in blood from victims of the Dupont Plaza Hotel fire in Puerto Rico bacteria 6 facts order 12 mg stromectol fast delivery. Experimental cyanide encephalopathy: Gradients of susceptibility in the corpus callosum. Spectroscopic characterization of the interaction of azide and thiocyanate with the binuclear center of cytochrome oxidase: Evidence for multiple ligand sites. Determination of thiocyanate metabolite of sodium nitroprusside in serum by spectrophotometry. Enhancement of cyanide-induced mitochondrial dysfunction and cortical cell necrosis by uncoupling protein-2. Oxidative stress and cyclooxygenase-2 induction mediate cyanide-induced apoptosis of cortical cells. High-performance liquid chromatographic determination of thiocyanate anions by derivatization with pentafluorobenzyl bromide. Fire in the environment: the ecological, atmospheric and climatic importance of vegetation fires. Determination of the cyanide metabolite 2aminothiazoline-4-carboxylic acid in urine and plasma by gas chromatography-mass spectrometry. The role of hydrogen cyanide and carbon monoxide in fire casualties: A prospective study. Flow-injection determination of cyanide by detecting an intermediate of the pyridine-barbituric acid chromogenic reaction. State influences on ventral medullary surface and physiological responses to sodium cyanide challenges. Protein kinase C modulation of rhodanese catalyzed conversion of cyanide to thiocyanate. Cyanide-induced alteration of cytosolic pH: Involvement of cellular hydrogen ion handling processes. Cyanide-induced neurotoxicity: Calcium mediation of morphological changes in neuronal cells. Reversed-phase liquid chromatographic determination of cyanide as 1-benzoyl-1,2-dihydroquinaldonitrile. Acute cyanide intoxication treated with a combination of hydroxycobalamin, sodium nitrite, and sodium thiosulfate. Mitochondrial toxin inhibition of [3H]dopamine uptake into rat striatal synaptosomes. Improved gas chromatography with electron-capture detection using a reaction pre-column for the determination of blood cyanide: A higher content in the left ventricle of fire victims. Protective effect of diltiazem on cyanide-induced neurotoxicity in Wistar strain rats. Role of calcium ions in dopamine release induced by sodium cyanide perfusion in rat striatum. Drugs during pregnancy and lactation: Handbook of prescription drugs and comparative risk assessment: With undated information on recreational drugs. Spectrophotometric determination of total cyanide, iron-cyanide complexes, free cyanide and thiocyanate in water by a continuous-flow system. Thiosulphate and hydroxocobalamin prophylaxis in progressive cyanide poisoning in guinea-pigs. Comparative efficacy of three methemoglobin formers in delaying effects of infused sodium cyanide. Lactic acidosis caused by sodium nitroprusside in a newborn with congenital heart disease. Differential susceptibility of brain areas to cyanide involves different modes of cell death. Volatilization of carbonyl sulfide from paddy soils treated with sulfurcontaining substances. Mantakassa: An epidemic of spastic paraparesis associated with chronic cyanide intoxication in a cassava staple area of Mozambique. Determination of thiocyanate in human urine samples by suppressed ion chromatography. An outbreak of acute intoxications from consumption of insufficiently processed cassava in Tanzania. Determinants of cyanide exposure from cassava in a konzo-affected population in northern Tanzania. Plasma thiocyanate and vitamin B12 in Nigerian patients with degenerative neurological disease. Measuring the mitotic index in chemically-treated human lymphocyte cultures by flow cytometry. Antidotal efficacy of vitamin B12 (hydroxocobalamin) in experimental cyanide poisoning. Bcl-2 protects neural cells from cyanide/aglycemia-induced lipid oxidation, mitochondrial injury, and loss of viability. Changes in the parameters of oxygen metabolism in a clinical course recovering from potassium cyanide. National Air Toxics Information Clearinghouse, Office of Air Quality Planning and Standards, U. Prevention of cyanide induced cytotoxicity by nutrients in isolated rat hepatocytes. Biosensors based on bilayer lipid membranes for automated continuous monitoring or rapid screening of environmental pollutants. Flow injection amperometric determination of cyanide on a modified silver electrode. Department of Health, Education, and Welfare, Center for Disease Control, National Institute for Occupational Safety and Health. Department of Health and Human Services, Public Health Services, Centers for Disease Control, National Institute for Occupational Safety and Health, Division of Standards Development and Technology Transfer. Protective effects of Kamikihi-to, a traditional Chinese medicine against cerebral ischemia, hypoxia and anoxia in mice and gerbils. Indirect determination of cyanide compounds by ion chromatography with conductivity measurement. Department of Health and Human Services, Public Health Service, National Institutes of Health. Regional potassium distribution in the brain in forensic relevant types of intoxication: Preliminary morphometric evaluation using a histochemical method. The cardiotoxicity of hydrogen cyanide as a component of polymer pyrolysis smokes. Spectrophotometric determination of total cyanide in surface waters following ultraviolet-induced photodecomposition. Formation of cyanogen chloride during the chlorination of water containing aromatic compounds and ammonium ion. Effects of cassava processing methods on antinutritional components and health status of children. Occupational and dietary exposures of humans to cyanide poisoning from large-scale cassava processing and ingestion of cassava foods. Excretion of 14C-labeled cyanide in rats exposed to chronic intake of potassium cyanide. Hypocholesterolemic and hypertriglycerolemic effects of chronic cyanide intoxication in rabbits. Some histological and biochemical evidence for mitigation of cyanideinduced tissue lesions by antioxidant vitamin administration in rabbits. Liver and kidney lesions and associated enzyme changes induced in rabbits by chronic cyanide exposure. Differential effects of chronic cyanide intoxication on heart, lung and pancreatic tissues. Safety and health regulations for construction: Gases, vapors, fumes, dusts, and mists. An ataxic neuropathy in Nigeria: A clinical, biochemical and electrophysiological study. Chronic cassava toxicity: Proceedings of an interdisciplinary workshop London, England, 29-30 January 1973. A degenerative neuropathy with blindness and chronic cyanide intoxication of dietary origin: the evidence in the Nigerians. Relationship of a degenerative tropical neuropathy to diet report of a field survey. Influence of age, sex and nutrition on body composition during childhood and adolescence. Dexamethasone induces resistance to the lethal consequences of electron transport inhibition in cultured hepatocytes. Cyanide induces Ca2+-dependent and independent release of glutamate from mouse brain slices. Blockade of N-methyl-D-aspartate receptors prevents cyanideinduced neuronal injury in primary hippocampal cultures. N-Methyl-D-aspartate receptors mediate cyanide-induced cytotoxicity in hippocampal cultures. Is there an energy conservation system in brain that protects against the consequences of energy depletion? Reversal of cyanide inhibition of cytochrome c oxidase by the auxiliary substrate nitric oxide. The effects of cyanide on brain mitochondrial cytochrome oxidase and respiratory activities. Simplified colorimetric determination of thiocyanate in biological fluid and its application of investigation of toxic amblyopias. Microdiffusion method for estimation of cyanide in whole blood and its application to the study of conversion of cyanide to thiocyanate. Urinary thiocyanate levels as a biomarker for the generation of inorganic cyanide from benzyl cyanide in the rat. Up-regulation of uncoupling protein 2 by cyanide is linked with cytotoxicity in mesencephalic cells. Cigarette smoking and serum levels of alpha-1 fetoprotein carcinoembryonic antigen cancer antigens 125 and 19-9 neurone-specific enolase. Acute effects of acetyl-L-carnitine on sodium cyanideinduced behavioral and biochemical deficits. Mitochondrial dysfunction and energy depletion from subchronic peroral exposure to cyanide using the Wistar rat as a mammalian model. Sodium nitroprusside metabolism in children during hypothermic cardiopulmonary bypass. A bioassay model for testing the incapacitating effects of exposure to combustion product atmospheres using cynomolgus monkeys. Interference in glucose and other clinical chemistry assays by thiocyanate and cyanide in a patient treated with nitroprusside. The effects of cyanide and its interactions with norepinephrine on isolated aorta strips from the rabbit, dog, and ferret. Determination of cyanide, sulfide, iodide, and bromide by ion chromatography with electrochemical detection. Biosensors for rapid monitoring of primary-source drinking water using naturally occurring photosynthesis. Neurological sequelae of cyanide intoxication - the patterns of clinical, magnetic resonance imaging, and positron emission tomography findings. A method development for the routine analytical monitoring of aqueous cyanide species. Co-oxidation of acrylonitrile by soybean lipoxygenase and partially purified human lung lipoxygenase. Determination of cyanide using a microdiffusion technique and potentiometric measurement. Effects of protein-free diet and food deprivation on hepatic rhodanese activity, serum proteins and acute cyanide lethality in mice. Cyanide overdose: Survival with fatal blood concentration without antidotal therapy. Phospholipid metabolism and intracellular Ca2+ homeostasis in cultured rat hepatocytes intoxicated with cyanide. Metabolic, cardiovascular, and neurologic aspects of acute cyanide poisoning in the rat. Spectrofluorometric determination of cyanide in blood and urine with naphthalene-2,3-dialdehyde and taurine. High-performance liquid chromatographic determination of cyanide in human red blood cells by pre-column fluorescence derivitization. Comparison of methemoglobin formers in protection against the toxic effects of cyanide. Antagonism of experimental cyanide toxicity in relation to the in vivo activity of cytochrome oxidase. Cyanide and Environment: Proceedings of a conference Tucson, Arizona December 11-14, 1984: Volume 1. The suppression of potassium cyanide-induced mortality by the increase of extracellular acetylcholine level in the brain.
The greatest percent error is likely to be associated with velocity values at low substrate concentration bacteria animation discount stromectol 3 mg. Hence the experimental error is amplified and unevenly weighted in this analysis bacteria kingdom characteristics buy generic stromectol 12 mg on-line, resulting in poor estimates of the kinetic constants even when the experimental error is relatively small bacteria definition for kids buy generic stromectol 3 mg on-line. To illustrate this antibiotics for uti and bv stromectol 12mg without prescription, let us compare the estimates of V and K obtained for the data in Table 5 antibiotics z pack dosage cheap stromectol 12 mg online. The fitting of the untransformed data to the Michaelis-Menten equation provided estimates of 100 antibiotic ophthalmic ointment safe stromectol 12mg. The errors are even greater when the double-reciprocal plots are used for the full data set in Table 5. The foregoing example, should convince the reader of the limitations of using linear transformations of the primary data for determining the values of the kinetic constants. Nevertheless, the Lineweaver-Burk plots are still commonly used by many researchers and, as we shall see in later chapters, are valuable tools for certain purposes. In these situations (described in detail in Chapters 8 and 11), we make the following recommendation. Rather than using linear regression to fit the reciprocal data in Lineweaver-Burk plots, one should determine the values of V and K by nonlinear regression analysis of the untransformed data fit to the Michaelis-Menten equation. Note the strong influence of the data points at low [S] (high 1/[S] values) on the best fit line from linear regression. The line drawn by this method may not appear to fit the reciprocal data as well as a linear regression fit, but it will be a much more accurate reflection of the kinetic behavior of the enzyme. The use of this method will be more clear when it is applied in Chapters 8 and 11 to studies of enzyme inhibition and multisubstrate enzyme mechanisms, respectively. If one is to ultimately present experimental data in the form of a doublereciprocal plot, it is desirable to chose substrate concentrations that will be evenly spaced along a reciprocal x axis. One picks a maximum value of [S] ([S]) to work with and makes a stock solution of substrate that will give this final concentration after dilution into the assay reaction mixture. Additional initial velocity measurements are then made by adding the same final volume to the enzyme reaction mixture from stock substrate solutions made by diluting the original stock solution by 1:2, 1:3, 1:4, 1:5, and so on. In this way, the data points will fall along the 1/[S] axis at intervals of 1, 2, 3, 4, 5. For example, let us say that we have decided to work with a maximum substrate concentration of 60 M in our enzymatic reaction. If we prepare a 600 M stock solution of substrate for this data point, we might dilute it 1:10 into our assay reaction mixture to obtain the desired final substrate concentration. Again, the use of these transformation methods is no longer necessary because most researchers have access to computer-based nonlinear curve-fitting methods, and the direct fitting of untransformed data by these methods is highly recommended. For the sake of historic persepective, however, we shall describe three other popular graphical methods for presenting enzyme kinetic data: Eadie-Hofstee, Hanes-Wolff, and Eisenthal-CornishBowden direct plots. These linear transformation methods, which are here applied to enzyme kinetic data, are identical to the Wolff transformations described in Chapter 4 for receptor-ligand binding data. In this plot the slope is 1/V, the y intercept is K /V, and the x intercept is K. For each pair, one then draws a straight line connecting the points on the two axes and extrapolates these lines past their point of intersection (Figure 5. When a horizontal line is drawn from the point of intersection of these line to the y axis, the value at which this horizontal line crosses the y axis is equal to V. Hence they are highly recommended when it is desired to determine these kinetic parameters but nonlinear curve fitting to Equation 5. If one is limited to measurements in which the substrate concentration is much less than the K, the reaction will follow pseudo-first-order kinetics, and it may be difficult to find a time window over which the reaction velocity can be approximated by a linear function. Even if quasi-linear progress curves can be obtained, a plot of initial velocity as a function of [S] cannot be used to determine the individual kinetic constants k and K, since the substrate concentration range that is experimentally attainable is far below saturation (as in Figure 5. In such situations one can still derive an estimate of k /K by fitting the reaction progress curve to a first-order equation at some fixed substrate concentration. Suppose that we were to follow the loss of substrate as a function of time under first-order conditions. However, occasional deviations from the hyperbolic dependence of velocity on substrate concentration are seen. Some physical methods of measuring velocity, such as optical spectroscopies, can lead to experimental artifacts that have the appearance of deviations from the expected behavior, and we shall discuss these in detail in Chapter 7. Nonhyperbolic behavior can also be caused by the presence of certain types of inhibitor as well. At low substrate concentrations, the kinetics follow simple Michaelis-Menten behavior. Above a critical substrate concentration, however, the data deviate significantly from the expected behavior. The binding of the second, inhibitory, molecule of substrate can be accounted for by the following equation: v: [S] [S] K; [S] 1; K V (5. Inhibition effects at very high substrate concentrations also can be readily detected as nonlinearity in the Lineweaver-Burk plots of the data. Here one observes a sudden and dramatic curving up of the data near the y-axis intercept. Another cause of nonhyperbolic kinetics is the presence of more than one enzyme acting on the same substrate (see also Chapter 4, Section 4. Many enzyme studies are performed with only partially purified enzymes, and many clinical diagnostic tests that rely on measuring enzyme activities are performed on crude samples (of blood, tissue homogenates, etc. If, however, the sample contains more than one enzyme that can act on the substrate, deviations from the expected kinetic results occur. Suppose that our sample contains two enzymes; both can convert the substrate to product, but they display different kinetic constants. Suppose further that for one of the enzymes V = V and K = K, and for the second enzyme V = V and K = K. One last example of deviation from hyperbolic kinetics is that of enzymes displaying cooperativity of substrate binding. As we saw in Chapter 3 and 4, sometimes proteins occur as multimeric assemblies of subunits. It is possible that the binding of a substrate molecule at one of these active sites could influence the affinity of the other active sites in the multisubunit assembly (see Chapter 12 for more details). It is said to be positive when the binding of a substrate molecule to one active site increases the affinity for substrate of the other active sites. On the other hand, when the binding of substrate to one active site lowers the affinity of the other active sites for the substrate, the effect is called negative cooperativity. The number of potential substrate binding sites on the enzyme and the degree of cooperativity among them can be quantified by the Hill coefficient, h. The influence of cooperativity on the measured values of velocity can be easily taken into account by modifying Equation 5. The velocity data for cooperative enzymes can be presented in a linear form by use of Equation 5. Thus, a plot of log(v/(V v)) as a function of log[S] should yield a straight line with slope of h and a y intercept of log(K), as illustrated in Figure 5. The utility of such plots is limited, however, by the need to know V a priori and because the linear relationship described by Equation 5. Hence, whenever possible, it is best to determine V, h, and K for cooperative enzymes from direct nonlinear curve fits to Equation 5. The slope of the best fit line provides an estimated of the Hill coefficient h, and the y intercept provides an estimate of log(K). A more comprehensive discussion of such deviations can be found in the texts by Segel (1975) and Bell and Bell (1988). The steady state kinetic constants k and K are complex functions that combined rate constants from multiple steps in the overall enzymatic reaction. Hence, these constants do not provide rate information on any individual steps in the reaction pathway. A steady state kinetic study would define the overall reaction in terms of k and K but would not provide much information on the rates and nature of the individual steps in the reaction. For example, in Chapter 11 we shall see how steady state kinetic measurements can define the order of substrate binding and product release for multisubstrate enzymes. These studies do not, however, give information on the specific reactions of the various enzyme species involved in catalysis, nor can they identify the rate-limiting step. To overcome some of these limitations, researchers turn to rapid kinetic methods that allow them to make measurements on a time scale. These methods are collectively referred to as transient state kinetics, and their application to enzymatic systems provides much richer kinetic detail than simple steady state measurements (Johnson, 1992). A variety of instruments have been designed for this purpose (Fersht, 1985), but the two most commonly used methods for measuring transient kinetics are stopped-flow and rapid reaction quenching. In a stopped-flow experiment, the researcher is measuring the formation of a transient species by detecting a unique optical signal (typically absorbance or fluorescence). The instrument consists of two syringes, one holding a enzyme solution and the other holding a substrate solution. Both syringes are attached to a common drive bar that compresses the plungers of both syringes at a steady and common rate, forcing the solutions from each syringe to mix in the mixing chamber and flow through the detection tube. As liquid is forced into this third syringe, its plunger is pushed back until it is stopped by contact with a stopping bar. The stopping bar has attached to it a microswitch, which triggers the controlling computer to initiate observation of the optical signal from the solution trapped in the detection tube. Measurements of the optical signal are then made over time as the solution ages in the detection tube. These solutions flow into the first mixing chamber and then through a reaction aging tube to the second mixing chamber. The length of reaction time is controlled by the length of the reaction aging tube, or by the rate of flow through this tube. In the second mixing chamber, the reaction mixture is combined with a third solution containing the quenching material, which rapidly stops (or quenches) the reaction. The quenching solution must be able to instantaneously stop the reaction by denaturing the enzyme or sequestering a critical cofactor or other component of the reaction mixture. For example, strong acids are commonly used to quench enzyme reactions in this way. Once the reaction has been quenched, the mixed solution flows into a collection container, from which it can be retrieved by the scientist. Detection is performed off-line by any convenient method, including spectroscopy, radiometric chromatography (including thin-layer chromatography), or electrophoretic separation of substrates and products (see Chapter 7 for details). With instruments like stopped-flow and rapid quench apparatus, one can measure the formation of products or intermediates on a millisecond time scale. Hence, the observed rate of formation k will depend on substrate concentration as follows: k: k [S]; k (5. The reader should be aware of the power of these methods for determining individual rate constants and of the value of such information for the development of detailed mechanistic models of catalytic turnover. Because of space limitations, and because these methods require specialized equipment that beginners may not have at their disposal, we shall suspend further discussion of these methods. Several noteworthy reviews on the methods of transient kinetics (Gibson, 1969; Johnson, 1992; Fierke and Hammes, 1995) are highly recommended to the reader who is interested in learning more about these techniques. These methods provide important kinetic and mechanistic information, mainly in the form of two kinetic constants, k and K. Graphical methods for determining the values for these kinetic constants were presented. We also briefly discussed the application of rapid kinetic techniques to the study of enzymatic reactions. These methods provide even more detailed information on the individual rate constants for different steps in the reaction sequence, but they require more specialized instrumentation and analysis methods. The chapter provided references to more advanced treatments of rapid kinetic methods to aid the interested reader in learning more about these powerful techniques. Together these properties of rate acceleration and substrate specificity afford enzymes the ability to perform the chemical conversions of metabolism with the efficiency and fidelity required for life. In this chapter we shall see that both substrate specificity and rate acceleration result from the precise three-dimensional structure of the substrate binding pocket within the enzyme molecule, known as the active site. Enzymes are (almost always) proteins, hence the chemically reactive groups that act upon the substrate are derived mainly from the natural amino acids. The identity and arrangement of these amino acids within the enzyme active site define the active site topology with respect to stereochemistry, hydrophobicity, and electrostatic character. Together these properties define what molecules may bind in the active site and undergo catalysis. The active site structure has evolved to bind the substrate molecule in such a way as to induce strains and perturbations that convert the substrate to its transition state structure. This transition state is greatly stabilized when bound to the enzyme; its stability under normal solution conditions is much less. Since attainment of the transition state structure is the main energetic barrier to the progress of any chemical reaction, we shall see that the stabilization of the transition state by enzymes results in significant acceleration of the reaction rate. We discussed in Chapter 4 the main forces involved in stabilizing protein-ligand interactions: hydrogen bonding, hydrophobic forces, van der Waals interactions, electrostatic interactions, and so on. All these contribute to the overall binding energy of the complex and must more than compensate for the lose of rotational and translational entropy that accompanies binary complex formation. It is clear today that formation of an enzyme-substrate binary complex is but the first step in the catalytic process used in enzymatic catalysis. Hence, a minimalist view of enzyme catalysis is captured in the scheme illustrated in Figure 6. While the active site of every enzyme is unique, some generalizations can be made: 1. The active site is three-dimensional - that is, amino acids and cofactors in the active site are held in a precise arrangement with respect to one another and with respect to the structure of the substrate molecule. This active site three-dimensional structure is formed as a result of the overall tertiary structure of the protein.
Generic stromectol 3mg without a prescription. 2019 NHSN LTCF Training - Antibiotic Stewardship in LTCF.
References