




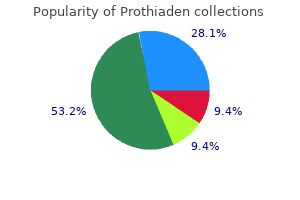
|
STUDENT DIGITAL NEWSLETTER ALAGAPPA INSTITUTIONS |

|
Jay Horrow, MD, MS, FAHA
A bruit may also be heard over a hepatoma because of increased flow with in the tumor treatment zoster ophthalmicus discount 75 mg prothiaden fast delivery. The Anus and Rectum: the left lateral position is best for routine examination of the rectum lanza ultimate treatment order prothiaden 75 mg amex. The examiner puts on a disposable pair of gloves treatment 4 pink eye buy 75mg prothiaden amex, informs the patient what he/she is about to do and does the examination as gently as possible symptoms 5 days after conception discount prothiaden 75mg otc. Note is made of any abnormalities of · the perianal skin the presence or absence of o perianal skin tags o perianal warts o fistula in ano o pilonidial sinus o anal fissure o perianal hematoma o prolapsed strangulated piles o perianal abscesses Digital examination is then carried out after putting a generous amount of lubricant on the gloved index finger of the right hand medicine 8 letters order 75 mg prothiaden otc. The pulp of the index finger is put flat on the anus 68 Physical Diagnosis and pressure applied firmly and slowly in a slightly backwards direction medicine ok to take during pregnancy cheap 75mg prothiaden with amex. After initial resistance the anal sphincter relaxes and the finger can be passed into the anal canal. Anal musculature tone is assessed, and the finger rotated 3600 in the canal to feel for any thickening or irregularity of the wall. The finger is then passed into the rectum and the rectal wall assessed with sweeping movements of the finger through 3600. With this maneuver, assess · texture of the wall area of tenderness irregularity of rectal mucosa presence of any mass, ulcers mobility of the rectal mucosa If you feel a mass at your fingertip, ask the patient to strain down. In men, the rectovesical pouch, seminal vesicles and the prostate should be felt anteriorly. In women, the cervix is felt as a firm, rounded mass projecting back into the anterior wall of the rectum. On withdrawing the finger after rectal examination, look for evidence of mucus, pus and blood on examining finger. This system is more dependent than most on laboratory, histopathology and imaging techniques for completion of the diagnostic process. The basic principles of clinical assessment, however, still apply; appropriate and careful history taking and physical examination are essential 69 Physical Diagnosis and can often lead to a diagnosis. A related group of signs and symptoms can arise in a patient with alterations of the sex organs. There is still unwarranted stigma and shame attached to sexually transmitted diseases. The interview and examination must be carried out in privacy and with confidentiality. As with other clinical problems, diagnosis is achieved by history, examination and relevant investigation. It may radiate into the lower quadrant of the abdomen and possibly to the upper thigh and testicle or labium Hematuria: Is the presence of red blood cells in the urine. Reddish discoloration of urine may be due to the presence of pigments in the urine. Oliguria: Denotes the passage of less than 400 ml of urine per day 70 Physical Diagnosis Anuria: Is the complete absence of urine output. Retention of urine should be excluded before a patient is considered to have anuria. It is an arbitrary definition, on the basis of 24 hours urine output of more than 3L per day. It results from polyuria or from a decrease in the functional bladder capacity as in bladder irritation or inflammation Nocturia: Implies the need to rise during hours of sleep to empty the bladder Dysuria: Is a specific form of discomfort arising from the urinary tract in which there is pain immediately before, during or immediately after micturation Urgency: Is the loss of the normal ability to postpone micturation beyond the time when the desire to pass urine is initially perceived Incontinence: Refers to an involuntary loss of urine that has become a social or hygienic problem Hesitancy: Is difficulty initiating the process of micturation Terminal dribbling: is difficulty of completing micturation in a clean stop fashion the Male Genital Tract Urethral discharge: is nearly always a complaint of men, in the form of o dripping o staining of the underwear the color, amount and duration of the discharge have to be ascertained. It can be grouped as: o Gonococcal urethritis o Non-gonococcal urethritis 71 Physical Diagnosis Genital ulcer: this may be recurrent, single or multiple, painful or painless. Common causes include o chancre of primary syphilis o chancroid o genital herpes Other complaints · History of sores, growths on the penis History of swelling or pain in the scrotum Past history of sexually transmitted infections History of sexual dysfunction the Female Genital Tract Vaginal discharge · can be associated with itching the color, odor and amount should be characterized Menstrual History: this part of history should be included. The history should include: · Date of last sexual contact, details of contacts over recent months, possibility of homosexual and bisexual contact and the type of sexual practice are among the things to be elicited upon history taking. Psychosexual problems, including erectile dysfunction and premature ejaculation, can present as complaints during history taking. Physical examination Urinary system Kidneys · Inspect the flanks for bruising or swelling Assess each kidney for tenderness. The size and consistency of the testis and any nodules or irregularities should be noted. Palpate for the spermatic cord; look for varicocele Prostate: Digital rectal examination will help assess the size, consistency, tenderness and invasion of the mucosa. A bivalve speculum is then inserted and the color of the vaginal wall inspected and discharges, if present, characterized with respect to color, odor and consistency. Having removed the speculum, the urethral orifice is examined for discharge, inflammation and warts. Digital examination o Lubricate the index and middle fingers of your gloved right hand. List down the factors that aid in differentiating an enlarged left kidney from an enlarged spleen. Mention the factors that aid in differentiating ureteric pain from a pin arising from the kidneys. Barbara Bates, A Guide to Physical Examination and History Taking, Sixth Edition, 1995 7. The location and relevance of lymph nodes to various pathologies the approaches in examination of lymph nodes Techniques how to examine the breast and the axillae And interpret the findings Introduction the lymph nodes are affected in many ways either directly or indirectly from diseases that originate in the lymphatic system itself or form any other organ system. The lymphatic circulation is an alternative circulation system in which heavy molecular weight substances are carried back to the circulation from tissues, and obviously, it also serves as a filtration in phagocytosis and immunological activities. The lymphatic drainage in a given tissue or organ system is initially to certain group of lymph nodes. The accessible lymph node groups in our body for physical examination are: Cervical lymph node groups Axillary lymph node groups Supraclavicular lymph node groups Inguinal lymph node groups Para aortic lymph node groups and others 78 Physical Diagnosis Cervical lymph node group: are affected usually by neck and face pathologies. They are also involved in systemic illness such as lymphomas, tuberculosis, and pyogenic infections. Lymphomas Hard or soft in consistency depending up on the pathology Small or big size Associated with discharge etc Patients complain of swelling in the neck, over the angle of the jaw or any where in the neck. It is therefore mandatory to be able to examine the nodes affected, as it is related to the understanding of the nature of the primary problem. Examination of the axillary lymph nodes: the Axillary lymph node groups are the commonly affected group by metastasis from breast carcinoma. Axillary lymph nodes are frequently involved in pathologies, neoplastic or inflammatory origin. Examination of axillary lymph nodes is done the patient being best in sitting position Pectoralis muscles should be relaxed, Examiner sitting on the same side of the axilla then Palpate systematically the five groups of lymph nodes. Examination of the inguinal lymph nodes: the inguinal lymph nodes are found along the inguinal canal. They often are affected from infection around the lower extremity and the external genitalia. Malignant diseases occurring in the scrotum and penis also affect this lymph node groups Other groups: Para aortic lymph nodes: these nodes are not usually accessible to physical examinations unless the patient is thin or wasted. Colorectal carcinoma metastasizes to these lymph nodes Pre trochlear nodes: Are located close to the elbow joint and affected by syphilis Examination of the Thyroid Gland Introduction the thyroid gland is located in the anterior neck attached to pretracheal fascia. It is composed of three lobes namely left lobe, right lobe and connecting the two lobes is the isthmus lobe. It is affected by lots of disorders, and in many cases it is enlarged, except in few conditions there may be simply changes in consistency. The most common pathology affecting the thyroid gland is iodine deficiency hyperplasia. However, other benign and malignant disorders could also produce enlargement of the thyroid gland. The following steps are followed as usual Inspection Size of the thyroid if it is enlarged, (minimally, moderately or severely) Movement with swallowing Any dominant or solitary nodule For scars, sinuses and change in color of the skin Palpation Palpation from in front (Fig 6. The lymphatic drainage of the breast is principally (> 75%) to the Ipsilateral axillary lymph nodes. History: Common breast complaints are: o lump in the breast o breast pain o nipple discharge and o ulceration Age: Different breast pathologies tend to occur in different age groups. Ask about: duration any accompanying nipple discharged multiply how it was first noticed change in size elation to menses: explanation Breast pain: It is mostly of functional and inflammatory origin. Ask about: o site, which quadrant o severity o associated swelling, lump, discharge o relation to menses (cyclic or non cyclic) o pregnancy, lactation 85 Physical Diagnosis Nipple discharge: Ask about: o color (bloody, serous, purulent, milky, etc) o spontaneous Vs non-Spontaneous o unilateral Vs bilateral o relation to menstrual cycle o associated breast lump o drug intake E. Oral contraceptives Ask for any risk factor for cancer o family history of breast cancer, 1st degree relation o age at menarche (<12 years) o age at menopause (>55 years) o nulliparity o history of contra lateral breast cancer Ask for symptoms of metastatic disease (if cancer is suspected) o bone pain or swelling o cough, dyspnea, hemoptysis o jaundice o neurological abnormalities Physical Examination General principles Should be done in a private place with good illumination Is more informative if done just after the end of menses patient should be in a semi sitting position expose the whole of the upper half of the body always start from the normal breast examine systematically, quadrant by quadrant Specific goals of examination are to: detect and characterize breast mass or masses 86 Physical Diagnosis elicit discharge from the nipple relate pain compliant to a specific breast finding detect skin changes detect enlarged axillary, supraclavicular or infraclavicular lymph nodes detect metastasis (If breast cancer suspected) Inspection: Stand in front of the patient. Palpation palpate with the palmar surface of your fingers roll the breast tissue between the chest wall and your hand palpate the whole breast quadrant by quadrant check for o skin temperature o consistency of breast, nodularity o tenderness o nipple discharge (expression) o mass discrete or indiscrete? Discuss the specific goals of examination of the breast 90 Physical Diagnosis Reference: 1. Barbara Bates, A Guide to physical Examination and History Taking, Sixth edition, 1995. Browse, An Introduction to the Symptoms and Signs of Surgical Disease, 3rd edition, 1997. The major part of the examination is therefore centered on the joint but must often be extended to include the nerves and muscles which are responsible for movement. This chapter reviews the principles of physical diagnosis related to the musculoskeletal system. It includes history and physical examination as both are needed in order to correctly evaluate diseases affecting the musculoskeletal system. The skill of interviewing patients to review the reason for presentation must be learned and constantly practiced by health workers. The history should be documented in chronologic order and include details of the current problem and all medical conditions before the present events. The age and sex of the patient can be significant in understanding the nature of the disorder and planning the treatment. If a patient complains of a thigh pain for example, the following questions should be raised and need to be understood in detail: Its specific location the character of the pain the frequency in which the pain occurs the duration and variation of the pain if any Aggravating or relieving factors Distribution or radiation of the pain to other locations Intensity and course should be determined. After as much as possible information about the present complaint(s), has been obtained, the history can be completed by obtaining the following: A review of the systems Family and social history the past history including a data about childhood illnesses, all immunizations, allergies and injuries All medical, surgical and obstetric treatments. Specific questions about the musculoskeletal system should include queries and elaborations on the following aspects: Pain Stiffness Swelling Buckling or the locking of a joint Deformity History of trauma including mechanism of injury After completing the history, the examiner should put the patient at ease and explain on the procedure for physical examination. A complete examination, checking all the systems, is needed to correctly diagnose a condition. Respecting the dignity, the patient should undress and put on a loose dressing that allows the examination of the entire body if necessary. These are summarized in the musculoskeletal examination as Look, Feel, Move, Measure and the Compare. Examination of the musculoskeletal system should begin by observing the general status of the patient on presentation. This may be helpful as the appearance of the patient and the way the patient moves can give some idea about the degree of apparent illness and whether or not in pain, about the nutritional and other state. When examining the limbs and joints, comparison to the contra lateral side should always be done in order to appreciate the abnormalities. This can be either due to disuse because of various joint or muscle problems or loss of nerve supply to the muscle. If there is a suggestion of muscle atrophy, compare each side of symmetric muscles by measuring the circumference at specific level. For example, the circumferences of the leg can be measured by arbitrarily measuring 10 cm below the tubercles of the tibia, which allows a comparison in the muscles of the legs. Tenderness the muscle under examination should be gently palpated for evidence of tenderness, especially when any part of a limb fails to move. In addition, the examiner must determine whether or not there is muscle spasm (a contraction of an individual muscle or muscle group) or relative weakness because of myositis or other local conditions. In relation to this, a muscle may have a normal, hypotonic or hypertonic state: Muscle hypotonia: this is a state which refers to a decreased tension or tone of a muscle with minimal or no resistance. The resistance of the muscle is maintained during passive movements and without collapse of the muscle. As listed below, the muscle power can numerically be graded according to the degree of contractility with grade zero indicating complete paralysis and grade five indicating full power of the muscle: 0= Zero No evidence of contractility 1= Trace or slight contractility or flicker, no motion with gravity eliminated 2= Poor Complete range of motion with gravity eliminated 3= Fair Complete range of motion against gravity 4= Good Complete range of motion against gravity with some resistance 5= Normal Complete range of motion against gravity with full resistance this method of grading will enable to establish the power grade of a muscle and recognize any changes. It also enables the examiner to differentiate organic diseases from other illnesses. A numerical value of 5 represents normal muscle power, while a decreasing grade indicates that power is correspondingly decreased. Starting the examination with a general inspection of the body with attention to the following aspects may be significant: the general movement of major joints the manner in which the patient stands or holds the extremity the general contour and length of the extremity in relation to the torso/trunk A comparison of each side for any noticeable difference 99 Physical Diagnosis During palpation of a joint, it should carefully be checked for warmth, swelling or tenderness and the findings should be recorded. In any event, the specific range of motion should be noted indicating the degree of abduction, adduction, flexion, and varus position for a given joint. Some joints also have special directions of motion like rotation of the hip, pronation and supination of the elbow, eversion and inversion of ankle. Moreover, data should be recorded for passive and active ranges of motion because both types of movement may provide important information in many instances. The general principles of measuring motion may be summarized as follows: 1) All motions should be measured by degrees from a neutral point zero. Once the testing for joint motions is done, the position of the joint needs to be described. Definition of some standard joint positions is described below: Abduction= Drawing away from the midline of the body Adduction= Draw towards the midline of the body Eversion= Turning outward Inversion= Turning inward Extension= the act of straightening, when the part distal to a joint extends 100 Physical Diagnosis Flexion= the act of bending, when the part distal to a joint bends. However, the common sequence listed below may be helpful and should be kept in mind. If the swelling is diffuse, does it seem confined to the joint or does it extend beyond the joint? Swelling confined to the joint suggests distension of the joint with: Excessive synovial fluid (effusion) E. If there is a localized swelling, note its position in relation to the underlying anatomic structures, as this may give a clue to its possible nature or identity.
Notifications regarding overdue evidence items signed out for temporary or long-term use are handled in a similar fashion and differ only in the time period between notifications (as identified in the chart below) treatment medical abbreviation buy prothiaden 75 mg low cost. At each interval symptoms just before giving birth order prothiaden 75mg with visa, notifications are sent to progressively higher management levels within the organization medications you can take while pregnant for cold prothiaden 75mg free shipping. Each agency must determine its own requirements for return of evidence signed out for investigative purposes symptoms gout generic 75 mg prothiaden mastercard. Just as with the tracking system medicine grace potter buy prothiaden 75 mg lowest price, the identification system can be simple or intricate medications made from plasma order prothiaden 75 mg otc. The key to any such system is that an identifier can never be duplicated and that the item of evidence can be correctly associated with a specific case. An example of such a method would be to assign a unique case number to unique item identifiers for each piece of evidence. The pre-numbered evidence tags/forms are controlled with a sign-out ledger that carefully tracks each evidence tag/form. The consecutively numbered tags/forms are similar to the management and tracking of traffic citations. In small agencies, evidence may be stored in only a few lockers, while in larger agencies, there may be many rooms or warehouses, and multiple physical locations. To easily retrieve an individual item of evidence or all of the evidence for a specific case, a tracking system must accurately and consistently provide the location of that evidence. Developing an intuitive scheme for evidence storage makes the system more manageable. It is critical that property room personnel update the tracking system with the new information if and when evidence is moved. If not updated, the tracking system will become useless and retrieval of evidence nearly impossible. Case Status Another key but often overlooked element to efficient and effective property rooms and tracking systems is the case status. For additional discussion of case status, refer to section V, page 38 Labels Proper labeling of evidence also is extremely important to a successful and efficient tracking effort. Minimally, the label should include the case identifier, item identifier, type of crime, date/time that the item was collected, where the item was collected, and the name or initials of the person who collected the item. It is also recommended that a description of the item in the package and biohazard labels be included, as appropriate. Any items that contain biological evidence are indicated as such either on the electronic property list or property record. Some use adhesive labels with or without barcodes while others may opt for pre-printed packaging. In all cases, the information should be readily available for as long as the evidence is maintained. Therefore, the following points should be considered: Are the label and information compatible with the tracking system? If evidence does not bear biological evidence labeling and the presence of biological evidence becomes known, the property and evidence tracking system and label need to be updated to indicate that biological material is contained. Electronic Evidence Management the increase in the volume of evidence, the budget-imposed decreases in resources available to manage evidence tracking, and the need to track evidence from the crime scene to the courtroom and through final disposition has increased interest and demand for more efficient systems to track and manage evidence. The cost of automated evidence tracking and management software is continually decreasing. Thus, evidence tracking and management software is becoming a "must" for an improved evidence processing system. Management system buyers should consider the following list, in consultation with relevant stakeholders, prior to buying an electronic management to match their process requirements: reporting capabilities (including statistics) tracking capabilities alert mechanisms ("tickler file") integration with existing systems security inventory management communication (enhancing data sharing with other criminal justice agencies) Technical Working Group on Biological Evidence Preservation 30 accessibility (web-based vs. Most evidence bagged and tagged at crime scenes is tracked manually by the responding personnel who fill out forms and hand-label the items collected. Automated systems can also be set up to send alerts to managers when highly sensitive evidence is moved. This shortcoming can be corrected by using a "tickler" or "flagging" system that indicates when evidence has not been returned by a predetermined time. Many agencies use more than one system to track evidence at different points in the process. The technology is changing rapidly, so considerations of factors such as integration with other systems and methods of accessing data-including web-based platforms-can influence a purchase decision. Workflow When choosing a tracking system, agencies must also consider their workflows. Important elements of forensic workflows include maintaining chain of custody, identifying all the data related to a case, and parent/child tracking. The systems available today have various capabilities and approaches to providing these capabilities. Report generation the final important consideration when selecting an evidence tracking and management system is its ability to generate management reports. A system must have the ability to search, run queries, and print and/or email the resulting data. For example, the ability to run an inventory report for each year in a 5year span could provide trend analysis that otherwise might be missed. The ability of the end-user, the property room staff members, laboratory staff members, or information technology staff members to customize system reports is a benefit of the more robust and capable systems. When transferring from manual to electronic tracking, agencies should procure or develop a system that can manage the entire process-from crime scene to disposition-and not just a portion of the process. As the cost of electronic tracking technologies drops and as integration between systems improves, many agencies that currently manage the forensic process manually will be able to justify purchasing more efficient electronic systems. Agencies need to review existing procedures, to conduct a needs assessment, to develop requirements, and to evaluate technology performance prior to procuring a system. Security Any location used to retain or store evidence must be secured to prevent tampering, contamination, theft, or contact with unauthorized people. Each evidence custodian should have an appropriate background check prior to employment or assignment to the unit. When evidence is transferred from one entity to another, public or private, it should either be hand carried or sent via a carrier that maintains an internal, detailed chain of custody with confirmed delivery. If evidence is opened for examination in a laboratory, during court proceedings, or for any other reason, it must be resealed prior to storing or transferring to another entity. Entities handling biological evidence should establish procedures that include steps to take if evidence is received unsealed. By establishing and following clear and concise procedures, the integrity of the evidence and the chain of custody will be kept intact. Courthouse Chain-of-Custody Procedures There are thousands of courthouses and courtrooms in the United States, and their procedures for tracking and maintaining a chain of custody and storing evidence vary. Because of the need to retain evidence post-trial, it is critical that courts follow guidelines for the storage of evidence. Evidence relevant to a proceeding may be stored and brought to the courtroom from an outside facility, a central property room within the courthouse, or a location designated by a judge. The evidence can be returned to any of these locations when it is no longer needed in the courtroom or when the proceedings are over for the session (Hampikian, West, and Akselrod 2011; Goray, van Oorschot, and Mitchell 2012; Daly, Murphy, and McDermott 2012; Lee, Crouse, and Kline 2010). When evidence is moved to the courthouse from another location, the courthouse should follow basic chain-of-custody requirements. These guidelines would apply during and between evidence viewings, pre-trial consultations, court proceedings, jury deliberations, and appellate and post-conviction reviews. Guidance on Possible Scenarios A record of how the evidence package is handled in the courtroom should be reflected in the court transcript. This would include any jury requests to see evidence that would result in changes to the packaging, such as unsealing and resealing. When possible and appropriate, exhibit numbers and case numbers should be cross-referenced in court proceedings. It is important to have designated locations for evidence storage, whether it is one centralized site for all the judges in a courthouse or a specific area for each judge. A trained court clerk or bailiff for all the judges or separate clerks for individual judges should safeguard the evidence and keep records using uniform procedures and paperwork. Procedures vary and lines of responsibility are not always clear regarding the repackaging, storage, and preservation of biological evidence after a verdict is rendered or a plea is entered. It is essential to carefully repackage and store evidence once trial court proceedings are completed, as the evidence may be requested again if there are appeals. To ensure the preservation of evidence post-conviction, it should be properly repackaged and returned as soon as possible to a designated storage site. The documentation accompanying the evidence package should be updated to record the transport back to storage. Hospitals should develop policies regarding the storage of biological evidence because the hospital and the individual collecting the evidence are involved in the chain of custody. The individual who collects the evidence from the patient is responsible for initiating the chain of custody process. According to the hospital accrediting body, Joint Commission on Accreditation of Healthcare Organizations, hospital staff members who are trained to identify abused patients should also know the procedures for preserving evidence that will support any future legal action. Guidance on Possible Scenarios If no law enforcement report is made at the time of the hospital/clinic visit, medical professionals should offer to collect evidence from a patient and to store the evidence until the patient or other appropriate person can decide if a police report will be filed. In many cases, there is no specified time period for Technical Working Group on Biological Evidence Preservation 34 which the facility will store the evidence. It is recommended that hospitals establish a specified time period for storage of biological evidence in consultation with the local prosecutor and/or police jurisdiction. If a law enforcement official does not request the evidence within the specified timeframe, the hospital should contact the patient and seek law enforcement agency authorization prior to destroying evidence. If the patient decides to file a report with a law enforcement representative, the medical facility may turn the evidence over directly to law enforcement. In this case, the law enforcement representative is required to sign the chain-of-custody form when taking custody of the evidence from a medical professional at the facility where the evidence was collected. If the patient has made a report and a law enforcement representative is not available to take custody of evidence, the medical facility can continue to store it or contact the relevant law enforcement agency to request that they handle the storage. When stored on hospital/clinic premises, dry evidence should be kept in a locked cabinet. Those with access can include sexual assault nurse examiners, sexual assault forensic examiners, and the charge nurse or designated supervisor at the medical facility. Evidence should be stored in a secure location requiring a signature for access and removal. It is not necessary for the same medical professional who collected the evidence to release it to law enforcement. The collector should document that he or she placed the evidence in a locked storage area. When a law enforcement representative comes to retrieve the evidence, the person at the medical facility who turns it over must indicate on the chain-of-custody form that he or she removed the evidence from storage and gave it to law enforcement. The law enforcement recipient also must sign for the evidence and note the time and date of the evidence transfer. Stakeholders should be informed of the following: the location of individual pieces of evidence, the status of each case as it pertains to the need for continued storage of evidence, and a consistent case identifier that all entities use and understand. The custodian should regularly audit property rooms to ensure adequate security measures are in place, proper evidence-handling procedures are practiced, and proper recordkeeping procedures are followed. Disposition is the ongoing process of determining what to do with evidence in a case. The process may entail retention and disposal, destruction, auction, diversion to governmental agency use, or return to owner. Case disposition includes the determination that the legal process is concluded, any further case investigation is completed, statutes of limitation have run for open cases, or no charges will be filed. A final evidence disposition is the permanent removal of evidence from inventory after the determination that the evidence is no longer required for any reason. The disposition process is accomplished by anyone responsible for the final determination of the need to retain evidence. This section discusses general practical considerations for the destruction, auction, or return to owner of biological evidence once the final determination is made that the evidence is no longer needed for any further purpose. Regardless of the age of the evidence, property and evidence custodians should follow these guidelines prior to the final disposition. This process may include getting a court order, receiving district attorney approval, notifying the law enforcement agency, and/or notifying the defendant/defense attorney or attorneys of record. Before any disposition, it is important to comply with existing laws, policies, regulations, and procedures. Specific detailed guidelines may be available in the applicable jurisdiction or through local, state, and international property organizations. Some evidence in the possession of a property and evidence custodian will pre-date a labeling system that mirrors the guidance in this handbook. The determination of what contains biological evidence in these circumstances should be made on a case-by-case basis and in accordance with the state policy/statute. Property and evidence custodians are responsible for locating this evidence if further identification is needed. A release-of-liability document should accompany the release of evidence to the lawful owner. Department policies and procedures need to address the elements of disposition of evidence. Almost all states that have evidence retention statutes also have mechanisms that authorize destruction prior to the regularly scheduled timeframe. These laws usually require that the holding agency provide advance notice to the court and all relevant parties. Some agencies can obtain criminal justice information electronically following the court process. Other holding agencies manually investigate to facilitate the flow of information to begin the disposition process. It is critical that the holding agency determine the status of the case and the requirements of the local evidence retention law prior to the disposition of evidence. The investigating officer authorizes release or disposal by making a note to that effect on the appropriate property form(s).
Do not infuse until confirmation of product release has been received from Dendreon; see Dilution medications memory loss buy prothiaden 75 mg fast delivery. Will be shipped directly to the infusing provider in packaging intended to protect the infusion bag and maintain storage temperatures until infusion medicine pouch trusted 75 mg prothiaden. Verify the product and patient-specific labels located on top of the insulated container 97110 treatment code prothiaden 75mg without a prescription. Do not remove from the shipping box or open the lid of the insulated container until the patient is ready for infusion treatment zone lasik 75mg prothiaden fast delivery. Do not administer if the bag leaks during handling or if clumps remain in the bag keratin treatment prothiaden 75mg line. Specific information not available; however symptoms 9dpiui cheap prothiaden 75 mg otc, specific use indicates it should be administered separately; consult pharmacist. If an infusion reaction occurs, decrease rate or interrupt infusion depending on the severity of the reaction; see Antidote. Neither is routinely tested for transmissible infectious diseases and thus may carry the risk of infectious disease transmission to health professionals during handling of the products. Incidence increased with the second infusion and decreased after the third infusion. If an acute infusion reaction occurs, the infusion may be interrupted or slowed depending on the severity of the reaction. Evaluate patients carefully for the medical appropriateness of reducing or discontinuing immunosuppressive agents before treatment. Severe acute infusion reactions (Grade 3) add asthenia, bronchospasm, dizziness, dyspnea, hypertension, hypoxia, and vomiting. For acute infusion reactions, the infusion may be interrupted or slowed depending on the severity of the reaction. Rapid or excessive administration may produce sodium overload, water retention, alkalosis, or hypokalemia. Hypothalamus osmoreceptors, sensitive to osmolarity changes in the blood, control serum sodium concentration (142 mEq/L). The acetate ion is metabolized to bicarbonate, thus providing a source of bicarbonate. Use with caution in impaired renal function, congestive heart failure, hypertension, peripheral or pulmonary edema, any condition resulting in salt retention, and in patients receiving corticosteroids. Hypernatremia, sodium level over 147 mEq/L, is most common (congestive heart failure, delirium, dizziness, edema, fever, flushing, headache, hypotension, oliguria, pulmonary edema, reduced salivation and lacrimation, respiratory arrest, restlessness, swollen tongue, tachycardia, thirst, weakness). Average dose for most indications is 2 to 5 mEq/kg/24 hr in adults and pediatric patients. Cardiac arrest: 1 mEq/kg of body weight, only when appropriate (see Precautions; evidence supports little benefit and use may be detrimental). For neonates and children up to 2 years of age, dose must never exceed 8 mEq/kg/24 hr of a 4. Rapid or excessive administration may produce alkalosis, hypernatremia, hypokalemia, and hypocalcemia. Best to partially correct acidosis and allow compensatory mechanisms to complete the correction. Maternal/Child: Category C: safety for use in pregnancy not established; use only if clearly needed. Rebreathing expired air from a paper bag may help to control beginning symptoms of alkalosis. Administration of a balanced hypotonic electrolyte solution (Isolyte H, Normosol-M, Plasma-lyte 56) with sodium and potassium chloride added may help to excrete the bicarbonate ion in the urine. For extravasation, discontinue infusion; aspirate fluid, drug, and/or 3 to 5 mL of blood through the in-place needle, then remove the needle. However, the amount of benzyl alcohol that is tolerated within 24 hours in adults without toxic effects has not been determined. To correct acute serious hyponatremia, hypertonic sodium chloride is used to correct the serum sodium in 5 mEq/L/dose increments at a rate of no more than 0. In the first 3 to 4 hours, an increase of plasma sodium at rates up to 1 mEq/L/hr may be tolerated in patients with distressing symptoms. To prevent an overly rapid correction, an increase in plasma sodium of less than 10 mEq/L in the first 24 hours and an increase of less than 18 mEq/L in the first 48 hours is desired. Start at the lower end of the desired dosing range; consider the greater frequency of decreased organ function and of concomitant disease or drug therapy. Isotonic and hypotonic sodium chloride are frequently combined with 5% or 10% dextrose. Permits specific mEq for mEq replacement of sodium and chloride without contributing to fluid overload. Bacteriostatic isotonic available in 2-mL, 10-mL, and 30-mL vials ready for use as a diluent. Too-rapid infusion may cause local pain and venous irritation; reduce rate for tolerance. Concentrated: Properly diluted in parenteral fluids and equally distributed over 24 hours. It controls water distribution Hypertonic: One-half the calculated dose over at least 8 hours. Do not exceed 100 mL over pendent on age, weight, and clinical condition of the patient. Body fluid is lost when sodium content decreases and retained when sodium content increases. Changes in the acid-base balance of the body are reflected by the changes in chloride concentration. Distribution and excretion of sodium and chloride are largely under control of the kidney, which maintains a balance between intake and output. Hypotonic: Water replacement without increase of osmotic pressure or serum sodium levels; treatment of hyperosmolar diabetes requiring considerable fluid without excess sodium. Hypertonic: Used only when high sodium and/or chloride content without large amounts of fluid is required. Concentrated: Used to meet the specific requirements of patients with unusual fluid and electrolyte needs. Hypernatremia; fluid retention; situations where sodium or chloride could be detrimental. Use caution in circulatory insufficiency, congestive heart failure, edema with sodium retention, kidney dysfunction, hepatic disease, hypoproteinemia, in the elderly or debilitated individuals, and in patients receiving corticosteroids. The risk of solute overload causing congested states with peripheral and pulmonary edema is directly proportional to the electrolyte concentration (higher electrolytes pull in fluid, leading to fluid overload). Monitor: Maintain accurate intake and output; monitor electrolytes and acid-base balance, especially in prolonged therapy. Maternal/Child: Category C: safety for use during pregnancy not established; use only if clearly needed. Safety and effectiveness based on similarity of clinical conditions of pediatric and adult populations. Use caution in neonates or very small infants; the volume of fluid may affect fluid and electrolyte balance. Elderly: Lower-end initial doses may be indicated in the elderly; see Dose Adjustments. Osmotic demyelination syndrome (central pontine myelinolysis) secondary to toorapid correction with hypertonic solutions. Excessive excretion of crystalloids to maintain normal osmotic pressure will increase excretion of potassium and bicarbonate and further increase acidosis. Sodium excess can be treated by sodium restriction and/or use of diuretics or hemodialysis to remove excessive amounts. Doses above 125 mg may be associated with a higher incidence and/or severity of adverse events; see Side Effects. May be administered over eight sessions at sequential dialysis treatments to achieve a favorable hemoglobin or hematocrit response. Additional doses as necessary are indicated to maintain target levels of hemoglobin, hematocrit, and laboratory parameters of iron storage within acceptable limits. Consider decreased cardiac, hepatic, or renal function, and concomitant disease or other drug therapy. Manufacturer states, "Do not mix with other medications or add to parenteral nutrition solutions. Infusion (adults and pediatric patients): A single dose as an infusion properly diluted and equally distributed over 60 minutes. Doses of 1 mg/day are adequate to replenish losses in healthy nonmenstruating adults. Treatment of iron deficiency anemia in adult patients and pediatric patients 6 years of age or older with chronic kidney disease who are receiving hemodialysis and supplemental erythropoietin therapy. The majority of these patients tolerated Ferrlecit therapy without a subsequent hypersensitivity reaction. Monitor: Recumbent position during and after injection may help to prevent postural hypotension. Hypotensive effects may be additive to transient hypotension during dialysis and/or from too-rapid rate of infusion; monitor closely. These symptoms may or may not be indicative of a hypersensitivity reaction and usually resolve within 1 or 2 hours. Doses in excess of iron needs may lead to accumulation of iron in iron storage sites and iatrogenic hemosiderosis. Maternal/Child: Category B: use during pregnancy only if potential benefit justifies potential risk to fetus. Benzyl alcohol present in maternal serum may cross into human milk and may be orally absorbed by a breast-feeding infant. Either postpone iron therapy to at least 24 hours after dimercaprol or consider transfusions. Hypotension associated with fatigue; light-headedness; malaise; severe pain in the chest, back, flanks, or groin; and weakness may be caused by too-rapid infusion. Most commonly reported side effects include abdominal pain, back pain, chest pain, cramps, diarrhea, dizziness, dyspnea, fever, headache, hypersensitivity reactions, hypertension, hypotension, infection, injection site reactions, nausea and vomiting, pain, pharyngitis, pruritus, rhinitis, tachycardia, and thrombosis. Many other side effects occurred in less than 1% of patients and may or may not be attributable to sodium ferric gluconate complex. Post-Marketing: Anaphylaxis, convulsions, dysgeusia, hypoesthesia, loss of consciousness, pallor, phlebitis, skin discoloration, and shock have been identified. May result in hemosiderosis, and excess iron may increase susceptibility to infection. If acute toxicity is seen, it may present as abdominal pain, diarrhea, or vomiting progressing to pallor or cyanosis, lassitude, drowsiness, hyperventilation due to acidosis, iatrogenic hemosiderosis, and cardiovascular collapse. Discontinue drug and treat hypersensitivity reactions or resuscitate as necessary; notify physician. Epinephrine (Adrenalin) and diphenhydramine (Benadryl) should always be available. A loading dose as an infusion is administered over 90 to 120 minutes, followed by an equivalent maintenance dose as an infusion administered over 24 hours. Non-protein calories should be supplied principally as glucose (8 to 10 mg/kg/min), with intravenous fat. Continue maintenance infusions until elevated plasma ammonia lev- els have been normalized or oral nutrition and medications can be tolerated. Loading dose: Dilute with an amount of D10W equal to or more than 25 mL/kg of body weight per dose. Equivalent maintenance dose: Also requires dilution with an amount of D10W equal to or more than 25 mL/kg of body weight per dose. A 4-kg neonate would require a minimum of 100 mL of D10W for dilution of the loading dose given over 90 to 120 minutes and a minimum of 100 mL of D10W for dilution of the maintenance dose given over 24 hours. An 80-kg adult would require a minimum of 2,000 mL of D10W for dilution of the loading dose over 90 to 120 minutes and a minimum of 2,000 mL D10W for dilution of the maintenance dose given over 24 hours. Manufacturer states, "Other infusion solutions and drug products should not be administered together with sodium phenylacetate and sodium benzoate. Loading dose: A single dose properly diluted as an infusion and equally distributed over 90 to 120 minutes. Maintenance dose: Each single dose properly diluted as an infusion and equally distributed over 24 hours. Two moles of nitrogen are removed per mole of phenylacetate when it is conjugated with glutamine, and one mole of nitrogen is removed per mole of benzoate when it is conjugated with glycine. Phenylacetate: Conjugates with glutamine in the liver and kidneys to form phenylacetylglutamine. It is then excreted by the kidneys via glomerular filtration and tubular secretion. The nitrogen content of phenylacetylglutamine per mole is identical to that of urea (2 moles of nitrogen). Plasma levels remain higher and are present for a longer period of time than plasma levels of benzoate. Benzoate: Conjugates with glycine to form hippuric acid, which is rapidly excreted by the kidneys by glomerular filtration and tubular secretion. The formation of hippurate from benzoate occurs more rapidly than that of phenylacetylglutamine from phenylacetate, and the rate of elimination of hippurate appears to be more rapid than that of phenylacetylglutamine. Adjunctive therapy for the treatment of acute hyperammonemia and associated encephalopathy in adult and pediatric patients with deficiencies in enzymes of the urea cycle. In acute hyperammonemic episodes, arginine supplementation, caloric supplementation, dietary protein restriction, hemodialysis, and other ammonia-lowering therapies should be considered. Uncontrolled hyperammonemia can rapidly result in brain damage or death, and prompt use of all therapies necessary to reduce ammonia levels is essential. Should be administered in a facility with adequate diagnostic and treatment facilities to monitor the patient and respond to any medical emergency. In addition, facilities for hemodialysis, nutritional support, and medical support are required.
The vaccines do not provide complete protection and should not replace hygiene precautions symptoms 16 weeks pregnant trusted 75mg prothiaden. Immunologicals A single dose of parenteral Vi capsular polysaccharide vaccine is recommended for adults and children over 2 years of age symptoms in dogs prothiaden 75mg overnight delivery, followed by booster doses every 3 years in cases of continued exposure medications available in mexico generic prothiaden 75 mg online. A live oral typhoid vaccine containing an attenuated strain of Salmonella typhi (Ty21a) is available either as enteric coated capsules symptoms uti in women prothiaden 75mg on-line, or as a liquid suspension symptoms xanax is prescribed for generic prothiaden 75 mg fast delivery. The capsules are licensed for individuals over 5 years of age and are given as 4 doses symptoms knee sprain discount 75 mg prothiaden overnight delivery, each 2 days apart; the suspension can be administered to children over 2 years of age and is given as 3 doses, each 2 days apart. In endemic areas, a booster dose of the live oral vaccine is recommended every 3 years; for travellers to endemic areas from non-endemic areas an annual booster is recommended. Inactivated whole-cell typhoid vaccines may still be available in some countries; children over 5 years of age are given 2 doses separated by an interval of 4 weeks, with a booster dose every 3 years. Capsule, live attenuated strain of Salmonella typhi (Ty21a) Oral suspension, live attenuated strain of Salmonella typhi (Ty21a) Injection, Vi capsular polysaccharide typhoid: 25 microgram/0. Administration of oral typhoid vaccine should be coordinated so that the antimalarial, mefloquine, is not taken for at least 12 hours before or after a dose; vaccination should be completed at least 3 days before the first dose of mefloquine or other antimalarials (except proguanil hydrochloride in combination with atovaquone, which may be given concomitantly). Oral typhoid vaccine is inactivated by concomitant administration of antibacterials; if possible antibacterials should be avoided 3 days before, or 3 days after, vaccination. Varicella vaccine Varicella-zoster (chickenpox) is a highly contagious disease caused by varicella-zoster virus. Various formulations of the live, attenuated vaccine based on the Okastrain are available. Varicella-zoster vaccine may be used as part of a national childhood immunization programme. The vaccine may also be used in adolescents or adults without a history of varicella but who are at increased risk of infection. A single dose of vaccine is effective in children aged 112 years; (however, the optimal age for immunization is 1224 months). In adults and adolescents over 13 years of age, 2 doses, each separated by 48 weeks, can be given. Post-exposure vaccination can be considered for seronegative health-care workers who come into direct contact with patients with varicella-zoster. Rarely, the varicella-zoster vaccine virus has been transmitted from vaccinated individuals to close contacts; if a vaccine-related rash develops within 46 weeks, contact with varicella-susceptible pregnant women and individuals at high risk of severe varicella infection, including patients with immunodeficiency or receiving immunosuppressive therapy, should be avoided. Contraindications: see introductory notes; also pregnancy (avoid pregnancy for 3 months after vaccination; Appendix 2); immunodeficiency; patients receiving immunosuppressive therapy; untreated active tuberculosis. Precautions: see introductory notes; also family history of congenital immune disorders. Adverse effects: see introductory notes; also mild varicella-like rash within 46 weeks. Immunologicals Yellow fever vaccine Yellow fever is a viral haemorrhagic fever which is endemic in tropical regions of Africa and South America. Yellow fever 17D vaccine is a live, attenuated vaccine, which offers protection from 10 days after vaccination, for at least 10 years. Yellow fever vaccine is also recommended for people at high risk of yellow fever exposure, including forestry and agricultural workers, and people living in or travelling to endemic areas. During epidemics, mass vaccination campaigns should be initiated as early as possible. Immunization is not recommended for infants aged 68 months or during pregnancy, except during an epidemic when the risk of transmission may be very high. Contraindications: see introductory notes; also not recommended for infants under 9 months of age. Precautions: see introductory notes; also pregnancy (Appendix 2); interactions: Appendix 1. Adverse effects: see introductory notes; also headache, myalgia, weakness; very rarely encephalitis (infants more susceptible); viscerotropic disease, multiple organ failure (the elderly more susceptible). Muscle relaxants (peripherally-acting) and cholinesterase inhibitors Muscle relaxants Muscle relaxants used in surgery are classified according to their mode of action as either depolarizing or non-depolarizing neuromuscular blocking drugs. They should never be given until it is certain that general anaesthesia has been established and ventilation must be mechanically assisted until they have been completely inactivated. It produces rapid, complete paralysis, which is very short lasting in most patients and is of particular value for laryngoscopy and intubation. Should paralysis be prolonged, ventilation must be assisted until muscle function is fully restored. Alcuronium is a non-depolarizing muscle relaxant with a duration of action of about 30 minutes. Its effects may be rapidly reversed after surgery by the anticholinesterase, neostigmine (see below), provided atropine (section 1. Vecuronium, another widely used non-depolarizing muscle relaxant, has a shorter duration of action (20 30 minutes); it causes minimal adverse cardiovascular effects. Cholinesterase inhibitors Reversal of block Cholinesterase inhibitors, such as neostigmine, are used at the end of an operation to reverse the muscle paralysis produced by non-depolarizing blocking drugs, such as alcuronium and vecuronium. Neostigmine must not be used with depolarizing blocking drugs, such as suxamethonium, since neostigmine will prolong the muscle paralysis. Neostigmine is also used to treat postoperative non-obstructive urinary retention. Myasthenia gravis Cholinesterase inhibitors, such as neostigmine and pyridostigmine, are used in the symptomatic treatment of myasthenia gravis. They act by inhibiting acetylcholinesterase, thereby prolonging the action of acetylcholine, and thus enhancing neuromuscular transmission; this produces at least a partial improvement in most myasthenic patients but complete restoration of muscle strength is rare. Unless the patient has difficulty in swallowing, cholinesterase inhibitors are given by mouth. Muscle relaxants (peripherally-acting) and cholinesterase inhibitors within 3060 minutes), but a longer duration of effect than neostigmine; it also tends to cause fewer muscarinic effects such as diarrhoea, abdominal cramps, and excess salivation, and so is usually preferred. Doses should be carefully adjusted to avoid precipitating a cholinergic crisis due to overdosage; this must be differentiated from a myasthenic crisis because of disease progression, and consequent underdosage; the principal effect in both cases is increased muscle weakness. In myasthenic crisis, if the patient has difficulty in breathing and in swallowing, the cholinesterase inhibitor must be given by intramuscular or subcutaneous injection; neostigmine is usually preferred over pyridostigmine in such cases. Precautions: renal impairment (Appendix 4); hepatic impairment (Appendix 5); burns patients (possibly increase dose); electrolyte disturbances; respiratory acidosis or hypokalaemia (possibly decrease dose); history of asthma; pregnancy (Appendix 2) and breastfeeding (Appendix 3); interactions: Appendix 1. Adverse effects: histamine release, leading to allergic reactions, such as wheal and flare effects at site of injection, flushing, and bronchospasm (anaphylactoid reactions reported); transient hypotension, slight increase in heart rate or decreased pulse rate. Muscle relaxants (peripherally-acting) and cholinesterase inhibitors Neostigmine Injection: 500 micrograms in 1-ml ampoule; 2. Contraindications: recent intestinal or bladder surgery; mechanical intestinal or urinary tract obstruction; after suxamethonium; pneumonia; peritonitis. Precautions: asthma; urinary tract infections; cardiovascular disease including arrhythmias (especially bradycardia, vagotonia, recent myocardial infarction or atrioventricular block); hyperthyroidism; hypotension; peptic ulcer; epilepsy; parkinsonism; renal impairment (Appendix 4); pregnancy (Appendix 2) and breastfeeding (Appendix 3); interactions: Appendix 1. Muscle relaxants (peripherally-acting) and cholinesterase inhibitors and micturition, miosis, nystagmus, bradycardia, heart block, arrhythmias, hypotension, agitation, excessive dreaming, and weakness eventually leading to fasciculation and paralysis; thrombophlebitis reported; rash associated tablet (bromide salt) formulations. Precautions: asthma; urinary tract infection; cardiovascular disease including arrhythmias (especially bradycardia or atrioventricular block); hyperthyroidism; hypotension; peptic ulcer; epilepsy; parkinsonism; avoid intravenous injection; renal impairment (Appendix 4); pregnancy (Appendix 2) and breastfeeding (Appendix 3); interactions: Appendix 1. Adverse effects: muscarinic effects generally weaker than those associated with neostigmine; and include increased salivation, nausea and vomiting, abdominal cramps, and diarrhoea; signs of overdosage include bronchoconstriction, increased bronchial secretions, lacrimation, excessive sweating, involuntary defecation and micturition, miosis, nystagmus, bradycardia, heart block, arrhythmias, hypotension, agitation, excessive dreaming, and weakness eventually leading to fasciculation and paralysis; thrombophlebitis; rash associated with tablet (bromide salt) formulations. Muscle relaxants (peripherally-acting) and cholinesterase inhibitors Suxamethonium Injection: 50 mg (chloride)/ml in 2-ml ampoule. Contraindications: inability to maintain clear airway; personal or family history of malignant hyperthermia; neurological disease involving acute wasting of major muscle, prolonged immobilization (risk of hyperkalaemia); personal or family history of congenital myotonic disease; Duchenne muscular dystrophy; myasthenia gravis; glaucoma, ocular surgery; liver disease; burns; low plasma cholinesterase activity (including severe liver disease); hyperkalaemia. Precautions: digitalis toxicity or recent digitalization; cardiac, respiratory or neuromuscular disease; paraplegia, spinal cord injury, or severe trauma; severe sepsis (risk of hyperkalaemia); prolonged apnoea on repeated injection (infusion preferred for long surgical procedures); hepatic impairment (Appendix 5); renal impairment (Appendix 4); pregnancy (Appendix 2) and breastfeeding (Appendix 3); children; interactions: Appendix 1. Adverse effects: postoperative muscle pain, particularly in patients ambulant after operation in females; myoglobinuria; myoglobinaemia; prolonged apnoea; increased intraocular pressure; hyperkalaemia; bradycardia, hypotension, and arrhythmias, particularly with halothane (but, with repeated doses, tachycardia, and hypertension); increased salivary, bronchial and gastric secretions; transient rise in intragastric pressure; hypersensitivity reactions including flushing, rash, urticaria, bronchospasm, and shock (more common in women, in history of allergy, or in asthmatics); rarely malignant hyperthermia (but often fatal). Muscle relaxants (peripherally-acting) and cholinesterase inhibitors Vecuronium Powder for injection: 10 mg (bromide) in vial. Contraindications: respiratory insufficiency or pulmonary disease; dehydrated or severely ill patients; myasthenia gravis or other neuromuscular disorders. Precautions: hepatic impairment (Appendix 5); burns patients (possibly increase dose); electrolyte disturbances; respiratory acidosis or hypokalaemia (possibly decrease dose); history of asthma; severe obesity (may require maintenance of adequate airway and ventilation support); pregnancy (Appendix 2) and breastfeeding (Appendix 3); interactions: Appendix 1. To avoid excessive dosage in obese patients, dose should be calculated on the basis of ideal body weight. Adverse effects: minimal release of histamine; rarely hypersensitivity reactions including bronchospasm, hypotension, tachycardia, oedema, erythema, and pruritus). Ophthalmological preparations Administration of eye preparations Preparations for use in the eye should be sterile when issued. Use of singleapplication containers is preferable; multiple-application preparations include the antimicrobial preservatives and when used particular care should be taken to prevent contamination of the contents, for example, by avoiding contact between the applicator and the eye or other surfaces. Eye drops are generally instilled into the lower conjunctival sac which is accessed by gently pulling down the lower eyelid to form a pocket into which one drop is instilled. The eye should be kept closed for as long as possible after application, preferably 12 minutes. A small amount of eye ointment is applied similarly; the ointment melts rapidly and blinking helps to spread it. When two different eye drops are required at the same time, dilution and overflow may occur if one immediately follows the other; an interval of at least five minutes should therefore be allowed between the two applications. Systemic absorption, which may occur after topical application of eye drops, can be minimized by using the finger to compress the lacrimal sac at the medial canthus for at least one minute after instillation of the drops. Performance of skilled tasks Application of eye preparations may cause blurring of vision which is generally transient; patients should be advised not to carry out skilled tasks, such as operating machinery or driving, until their vision has cleared. However, in some cases, for example, in gonococcal conjunctivitis, both topical and systemic anti-infective treatment may be necessary. Blepharitis and conjunctivitis are often caused by staphylococcus, while keratitis and endophthalmitis may be bacterial, viral, or fungal. Although most cases of acute bacterial conjunctivitis may resolve spontaneously, anti-infective treatment shortens the infectious process and prevents complications. Acute infective conjunctivitis is treated with antibacterial eye drops by day and eye ointment applied at night. Ophthalmological preparations is an antiviral used in the treatment of keratitis due to herpes simplex virus. Gentamicin is a broad-spectrum bactericidal aminoglycoside antibiotic with particular activity against Pseudomonas aeruginosa, Neisseria gonorrhoea and other bacteria that may be implicated in blepharitis or conjunctivitis. Tetracycline is a broad spectrum antibiotic with activity against many Grampositive and Gram-negative bacteria including N. Ophthalmic tetracycline is used in blepharitis, conjunctivitis, and keratitis produced by susceptible bacteria. Tetracycline is also used in the treatment of trachoma caused by Chlamydia trachomatis and in the prophylaxis of neonatal conjunctivitis (ophthalmia neonatorum) caused by N. Uses: keratitis caused by herpes simplex; systemic herpes simplex infections (section 6. Adverse effects: local irritation including transient mild stinging, inflammation; superficial punctuate keratopathy reported; very rarely hypersensitivity reactions including angioedema. Contraindications: hypersensitivity to aminoglycoside group of antibiotics Precautions: prolonged use may lead to skin sensitization and emergence of resistant organisms including fungi; discontinue if there is purulent discharge, inflammation or exacerbation of pain. Anti-inflammatory agents Ophthalmic corticosteroids should only be used under supervision of an ophthalmologist as inappropriate use is potentially blinding. Dangers include the development of open-angle glaucoma (chronic simple glaucoma) and cataracts, and the aggravation of a simple herpes simplex epithelial lesions into extensive corneal ulcers and subsequent permanent corneal scarring, with possible damage to vision and even loss of the eye. Corticosteroids such as prednisolone are useful in the treatment of inflammatory eye conditions including uveitis and scleritis. Before administration of an ophthalmic corticosteroid, the possibility of bacterial, viral, or fungal infection should be excluded. Treatment should be with the lowest effective dose for the shortest possible time; if long-term therapy (more than 6 weeks) is unavoidable, withdrawal of an ophthalmic corticosteroid should be gradual to avoid relapse. Precautions: cataract; corneal thinning, corneal or conjunctival infection; discontinue treatment if no improvement within 7 days; risk of adrenal suppression after prolonged use in infants. Adverse effects: secondary ocular infection; impaired corneal healing (due to corneal thinning), optic nerve damage, cataract; glaucoma, mydriasis, ptosis, epithelial punctate keratitis, delayed hypersensitivity reactions including burning, and stinging. Contraindications: hypersensitivity to ester-type local anaesthetics; eye inflammation or infection. Precautions: avoid prolonged use (risk of severe keratitis, permanent corneal opacification, scarring, and delayed corneal healing); protect eye from dust and bacterial contamination until sensation is fully restored. The rise in pressure is almost always due to reduced outflow of aqueous humour, the inflow remaining constant. The most common condition is chronic open-angle glaucoma (chronic simple glaucoma) in which the intraocular pressure increases gradually and the condition is usually asymptomatic until well advanced. In contrast, angle-closure glaucoma (closed-angle glaucoma) usually occurs as an acute emergency resulting from a rapid rise in intraocular pressure; if treatment is delayed, chronic angle-closure glaucoma may develop. In ocular hypertension, intraocular pressure is raised without signs of optic nerve damage. Ophthalmological preparations Drugs used in the treatment of glaucoma lower the intraocular pressure by a variety of mechanisms including reducing the secretion of aqueous humour by the ciliary body, or increasing the outflow of the aqueous humour by the opening of the trabecular network. Antiglaucoma drugs used include a betablocker (beta-adrenoceptor antagonist), a miotic, or a sympathomimetic such as epinephrine (section 21. Timolol is a non-selective beta-blocker that reduces the secretion of aqueous humour. A beta-blocker, applied topically, is usually the drug of choice for both initial and maintenance treatment of chronic open-angle glaucoma. If further reduction in intraocular pressure is required a miotic, a sympathomimetic, or a systemic carbonic anhydrase inhibitor may be used together with timolol.
Purchase prothiaden 75mg with amex. Pneumonia | Nucleus Health.
References