




|
STUDENT DIGITAL NEWSLETTER ALAGAPPA INSTITUTIONS |

|
Lawrence John Appel, M.D., M.P.H.

https://www.hopkinsmedicine.org/profiles/results/directory/profile/0001071/lawrence-appel
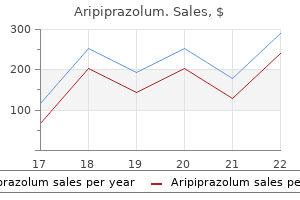
The drug(s) of choice within each family that is/are used for treating a specific bacterial infection are shown in bold print depression bipolar support alliance buy aripiprazolum 15 mg with mastercard, as illustrated for Staphylococcus aureus in Figure 30 anxiety chest pains buy 10 mg aripiprazolum with amex. An example of the bar chart with the drugs of choice for the treatment of Staphylococcus aureus shown in bold print vapor pressure depression definition chemistry safe 20 mg aripiprazolum. In this chapter depression symptoms chronic 15 mg aripiprazolum amex, the pie chart is used to illustrate the spectra of bacteria for which a particular class of antibiotics is therapeutically effective. Narrow-spectrum antibiotics Chemotherapeutic agents acting only on a single or a limited group of microorganisms are said to have a narrow spectrum. Extended-spectrum antibiotics Extended spectrum is the term applied to antibiotics that are effective against gram-positive organisms and also against a significant number of gram-negative bacteria. For example, ampicillin is considered to have an extended spectrum, because it acts against gram-positive and some gram-negative bacteria (Figure 30. Broad-spectrum antibiotics Drugs such as tetracycline and chloramphenicol affect a wide variety of microbial species and are referred to as broad-spectrum antibiotics P. Administration of broad-spectrum antibiotics can drastically alter the nature of the normal bacterial flora and precipitate a superinfection of an organism such as Candida albicans, the growth of which is normally kept in check by the presence of other microorganisms. Combinations of Antimicrobial Drugs It is therapeutically advisable to treat patients with the single agent that is most specific for the infecting organism. This strategy reduces the possibility of superinfection, decreases the emergence of resistant organisms (see below), and minimizes toxicity. Advantages of drug combinations Certain combinations of antibiotics, such as β-lactams and aminoglycosides, show synergism; that is, the combination is more effective than either of the drugs used separately. Disadvantages of drug combinations A number of antibiotics act only when organisms are multiplying. Thus, coadministration of an agent that causes bacteriostasis plus a second agent that is bactericidal may result in the first drug interfering with the action of the second. For example, bacteriostatic tetracycline drugs may interfere with the bactericidal effect of penicillins and cephalosporins. Drug Resistance Bacteria are said to be resistant to an antibiotic if the maximal level of that antibiotic that can be tolerated by the host does not halt their growth. However, microbial species that are normally responsive to a particular drug may develop more virulent, resistant strains through spontaneous mutation or acquired resistance and selection. Genetic alterations leading to drug resistance Acquired antibiotic resistance requires the temporary or permanent gain or alteration of bacterial genetic information. If the cell survives, it can replicate and transmit its mutated properties to progeny cells. However, mutations that produce antibiotic-resistant strains can result in organisms that may proliferate under certain selective pressures. An example is the emergence of rifampin-resistant Mycobacterium tuberculosis when rifampin is used as a single antibiotic. Resistance properties are usually encoded in extrachromosomal R factors (resistance plasmids). Plasmids may enter cells by processes such as transduction (phage mediated), transformation, or bacterial conjugation. Altered expression of proteins in drug-resistant organisms Drug resistance may be mediated by a variety of mechanisms, such as a lack of or an alteration in an antibiotic target site, lowered penetrability of the drug due to decreased permeability, increased efflux of the drug, or presence of antibiotic-inactivating enzymes (see Figure 30. Decreased accumulation: Decreased uptake or increased efflux of an antibiotic can confer resistance, because the drug is unable to attain access to the site of its action in sufficient concentrations to injure or kill the organism. For example, gram-negative organisms can limit the penetration of certain agents, including β-lactam antibiotics, tetracyclines, and chloramphenicol, as a result of an alteration in the number and structure of porins (channels) in the outer membrane. Enzymic inactivation: the ability to destroy or inactivate the antimicrobial agent can also confer resistance on microorganisms. Prophylactic Antibiotics Certain clinical situations require the use of antibiotics for the prevention rather than the treatment of infections (Figure 30. Because the indiscriminate use of antimicrobial agents can result in bacterial resistance and superinfection, prophylactic use is restricted to clinical situations in which the benefits outweigh the potential risks. Complications of Antibiotic Therapy Because the mechanism of action of a particular antibiotic is selectively toxic to an invading organism does not insure the host against adverse effects. Hypersensitivity Hypersensitivity reactions to antimicrobial drugs or their metabolic products frequently occur. For example, the penicillins, despite their almost absolute selective microbial toxicity, can cause serious hypersensitivity problems, ranging from urticaria (hives) to anaphylactic shock. Direct toxicity High serum levels of certain antibiotics may cause toxicity by directly affecting cellular processes in the host. For example, aminoglycosides can cause ototoxicity by interfering with membrane function in the hair cells of the organ of Corti. Superinfections Drug therapy, particularly with broad-spectrum antimicrobials or combinations of agents, can lead to alterations of the normal microbial flora of the upper respiratory, intestinal, and genitourinary tracts, permitting the overgrowth of opportunistic organisms, especially fungi or resistant bacteria. Sites of Antimicrobial Actions Antimicrobial drugs can be classified in a number of ways. These include 1) by their chemical structure (for example, β-lactams or aminoglycosides), 2) by their mechanism of action (for example, cell wall synthesis inhibitors), or 3) by their activity against particular types of organisms (for example, bacteria, fungi, or viruses). Chapters 31 through 33 are organized by the mechanisms of action of the drug, and Chapters 34 through 38 are organized according to the type of organisms affected by the drug (Figure 30. Prevention of meningitis among individuals in close contact with infected patients. The cell wall is composed of a polymer called peptidoglycan that consists of glycan units joined to each other by peptide cross-links. To be maximally effective, inhibitors of cell wall synthesis require actively proliferating microorganisms; they have little or no effect on bacteria that are not growing and dividing. The most important members of this group of drugs are the β-lactam antibiotics (named after the β-lactam ring that is essential to their activity) and vancomycin. Penicillins the penicillins are among the most widely effective antibiotics and also the least toxic drugs known, but increased resistance has limited their use. Members of this family differ from one another in the R substituent attached to the 6-aminopenicillanic acid residue (Figure 31. The nature of this side chain affects the antimicrobial spectrum, stability to stomach acid, and susceptibility to bacterial degradative enzymes (β-lactamases). Mechanism of action the penicillins interfere with the last step of bacterial cell wall synthesis (transpeptidation or cross-linkage 1), resulting in exposure of the osmotically less stable membrane. Cell lysis can then occur, either through osmotic pressure or through the activation of autolysins. Penicillins are only effective against rapidly growing organisms that synthesize a peptidoglycan cell wall. Consequently, they are inactive against organisms devoid of this structure, such as mycobacteria, protozoa, fungi, and viruses. Penicillin-binding proteins: Penicillins inactivate numerous proteins on the bacterial cell membrane. Exposure to these antibiotics can therefore not only prevent cell wall synthesis but also lead to morphologic changes or lysis of susceptible bacteria. Alterations in some of these target molecules provide the organism with resistance to the penicillins. Penicillins inhibit this transpeptidase-catalyzed reaction, thus hindering the formation of cross-links essential for cell wall integrity. Production of autolysins: Many bacteria, particularly the gram-positive cocci, produce degradative enzymes (autolysins) that participate in the normal remodeling of the bacterial cell wall. In the presence of a penicillin, the degradative action of the autolysins proceeds in the absence of cell wall synthesis. In general, gram-positive microorganisms have cell walls that are easily traversed by penicillins and, therefore, in the absence of resistance are susceptible to these drugs. Gram-negative microorganisms have an outer lipopolysaccharide membrane (envelope) surrounding the cell wall that presents a barrier to the water-soluble penicillins.
The systems and mechanisms involved in these effector functions are largely non-specific geriatric depression definition aripiprazolum 15 mg. Specific immune recognition by B and T cells directs these effector mechanisms to specific targets mood disorder ottawa 20mg aripiprazolum fast delivery. Usage subject to terms and conditions of license 86 2 Basic Principles of Immunology digestion anxiety 2 year old 10mg aripiprazolum with visa. Opsonization involves the coating of such microbes with Fc-expressing antibodies which facilitates their phagocytosis by granulocytes bipolar depression episodes aripiprazolum 20mg without a prescription. Many cells, particularly phagocytes (and interestingly enough also some bacteria like staphylococci), bear surface Fc receptors that interact with different Ig classes and subclasses. Mast cells and basophils bear IgE molecules, and undergo a process of degranulation following interaction with allergens against which the IgE molecules are directed. It is made up of a co-operative network of plasma proteins and cellular receptors, and is largely charged with the following tasks: & Opsonization of infectious pathogens and other foreign substances, with the aim of more efficient pathogen elimination. Bound complement factors can: enhance the binding of microbes to phagocytozing cells; result in the activation of inflammatory cells; mediate chemotaxis; induce release of inflammatory mediators; direct bactericidal effects; and induce cell lysis. The production of a C3 convertase, which splits C3 into C3a and C3b, is common to both pathways. C3b degradation products are recognized by receptors on B lymphocytes; they stimulate the production of antibodies as well as pathogen phagocytosis. The cleavage products C3a and C4a are chemotactic in their action, and stimulate expression of adhesion molecules. Component fragments are designated by small letters, whereby the first fragment to be split off (usually of low molecular weight) is termed "a" (e. Molecules often group to form complexes; in their designations the individual components are lined up together and are usually topped by a line. Usage subject to terms and conditions of license Immune Responses and Effector Mechanisms 87 & Solubilization of otherwise insoluble antigen-antibody complexes. Over 20 proteins of the complement system have been identified to date, and are classified as either activation or control proteins. C3 is not only present in the largest amount, but also represents a central structure for complement activation. Usage subject to terms and conditions of license 88 2 Basic Principles of Immunology Immunological Cell Death 2. During classic activation of complement, C1q must be bound by at least two antigen-antibody immune complexes, to which C4 and C2 then attach themselves. Pentameric IgM represents a particularly efficient C activator since at least two Ig Fc components in close proximity are required for C1q binding and activation. During alternative activation of complement, the splitting of C3 occurs directly via the action of products derived from microorganisms, endotoxins, polysaccharides, or aggregated IgA. C3b, which is produced in both cases, is activated by the factors B and D, then itself acts as C3 convertase. Usage subject to terms and conditions of license Immune Responses and Effector Mechanisms 89 and alternative activation, but is not necessarily essential since the released chemotaxins and opsonins are often alone enough to mediate the functions of microbe neutralization and elimination. Some viruses can activate the complement system without the intervention of antibodies by virtue of their ability to directly bind C1q. Importantly, without a stringent control mechanism complement would be activated in an uncontrolled manner, resulting in the lysis of the hosts own cells (for instance erythrocytes). Complement Control Proteins the following regulatory proteins of the complement system have been characterized to date: C1 inhibitor, prevents classic complement activation. This protein is lacking in patients suffering from paroxysmal nocturnal hemoglobinuria. This is a glycolipid anchored within the cell surface which prevents C9 from binding to the C5b-8 complex, thus protecting the cell from lysis. C5a initiates the chemotactic recruitment of granulocytes and monocytes, promotes their aggregation, stimulates the oxidative processes, and promotes the release of the thrombocyte activating factor. Immunological Tolerance & T-cell tolerance, as defined by a lack of immune reactivity can be due to a number of processes: Firstly, Negative selection in the thymus (referred to as deletion); secondly a simple lack of reactivity to antigen (self or nonself) as a result of the antigen having not been present in the secondary lymphoid organs in a sufficient quantity or for a sufficient amount of time; and thirdly an excessive stimulation of T-cells resulting from the ubiquitous presence of sufficient antigen resulting in T cell exhaustion. Finally, it may also be possible that T cells can become temporarily "anergized" by partial or incomplete antigen stimulation. As a general rule, self-reactive (autoimmune) B cells are not generally deleted by negative selection and can therefore be present in the periphery. Exceptions to this rule include B cells specific for membrane-bound self-determinants, some of which are deleted or anergized. B cells react promptly to antigens, even self-antigens, which are arranged repetitively. However, they only react to soluble monomeric antigens if they additionally receive T cell help. Thus, B-cell non-reactivity largely results from a lack of patterned antigen presentation structures or as a result & of T-cell tolerance. Immunological tolerance describes the concept that the immune system does not normally react to autologous structures, but maintains the ability to react against foreign antigens. Tolerance is acquired, and can be measured as the selective absence of immunological reactivity against specified antigens. T-Cell Tolerance A distinction can be made between central tolerance, which develops in the thymus and is based on the negative selection (deletion) of T cells recognizing self antigens present in the thymus, and peripheral tolerance. Usage subject to terms and conditions of license Immunological Tolerance 91 form of tolerance involves antigen recognition byantigen-reactive peripheral T cells, followed by a process of clonal cell proliferation, end differentiation and death. The following mechanisms have been postulated, and in some cases confirmed, to account for a lack of peripheral T-cell responsiveness (Table 2. Most self-antigens, not present in the serum or in lymphohematopoietic cells, belong to this category and are ignored despite the fact that they are potentially immunogenic. Certain viruses, and their antigens, actually take advantage of this system of ignorance. For instance, the immune system ignores the rabies virus when it is restricted to axons, and papilloma viruses as long as the antigens are restricted to keratinocytes (warts). The main reason why many self antigens, and some foreign antigens, are ignored by T cells is that immune responses can only be induced within the spleen or in lymph nodes, and non-activated (or naive) T cells do not migrate into the periphery. It has also been postulated that those naive T and B cells which do encounter antigens in the periphery will become anergized, or inactivated, due to a lack of the so-called costimulatory or secondary signals at these sites. Experiments seeking to understand the "indifference" of T cells are summarized in the box on p. In all probability, a great many self-antigens (as well as peripheral tumors) are ignored by the immune system in this way. During such a scenario the responding T cells differentiate into shortlived effector cells which only survive for two to four days. This induction phase may actually correspond to the postulated phenomenon of anergy (see Table 2. Should this be the case, anergy-defined as the inability of T cells to react to antigen stimulation in vitro-may in fact be explained by the responding cells having already entered a pathway of cell death (apoptosis). Once all the terminally differentiated effector T cells have died, immune reactivity against the stimulating antigen ends. Tolerance is hereafter maintained, as should the responsible antigen have entered into the thymus those newly maturing thymocytes will be subjected to the process of negative selection (e. Usage subject to terms and conditions of license 92 2 Basic Principles of Immunology iphery will continuously be induced to undergo activation and exhaustion within the secondary lymphoid organs. Successful establishment of lymphocyte chimerism following liver transplants appears to based on the same principle. Following sensitization of the skin flap with a contact antigen the animal reacted to a second antigenic exposure of the remaining (intact) skin with accelerated kinetics. When the lymph vessel leading from the prepared skin flap to the lymph node was interrupted, or the draining lymph node was destroyed prior to the initial sensitization, the typical secondary response was not observed-leading to the conclusion that no T cell response was induced. Following an initial sensitization at any other location on the skin the secondary response was observed, even on the skin flap regardless of interruption of the lymph vessel or destruction of the draining lymph node.
Cheap 15mg aripiprazolum with mastercard. Borderline Personality Disorder - BPD - (TEST).
Nevertheless mood disorder screening test trusted 20mg aripiprazolum, this does not involve anxiety young child purchase 15mg aripiprazolum visa, imply depression test had discount aripiprazolum 10mg with amex, or express any guarantee or responsibility on the part of the publishers in respect to any dosage instructions and forms of applications stated in the book depression existential crisis cheap aripiprazolum 15mg visa. Such examination is particularly important with drugs that are either rarely used or have been newly released on the market. The authors and publishers request every user to report to the publishers any discrepancies or inaccuracies noticed. Some of the product names, patents, and registered designs referred to in this book are in fact registered trademarks or proprietary names even though specific reference to this fact is not always made in the text. Therefore, the appearance of a name without designation as proprietary is not to be construed as a representation by the publisher that it is in the public domain. This applies in particular to photostat reproduction, copying, mimeographing, preparation of microfilms, and electronic data processing and storage. Usage subject to terms and conditions of license V Preface Medical Microbiology comprises and integrates the fields of immunology, bacteriology, virology, mycology, and parasitology, each of which has seen considerable independent development in the past few decades. The common bond between them is the focus on the causes of infectious diseases and on the reactions of the host to the pathogens. The objective of this textbook of medical microbiology is to instill a broadbased knowledge of the etiologic organisms causing disease and the pathogenetic mechanisms leading to clinically manifest infections into its users. This knowledge is a necessary prerequisite for the diagnosis, therapy, and prevention of infectious diseases. Beyond this academic purpose, its usefulness extends to all medical professions and most particularly to physicians working in both clinical and private practice settings. This book makes the vast and complex field of medical microbiology more accessible by the use of four-color graphics and numerous illustrations with detailed explanatory legends. Most chapters begin with a concise summary, and in-depth and supplementary knowledge are provided in boxes separating them from the main body of text. This textbook has doubtless benefited from the extensive academic teaching and the profound research experience of its authors, all of whom are recognized authorities in their fields. The authors would like to thank all colleagues whose contributions and advice have been a great help and who were so generous with illustration material. The authors are also grateful to the specialists at Thieme Verlag and to the graphic design staff for their cooperation. The Pathogens That Cause Gas Gangrene (Clostridial Myonecrosis) and Anaerobic Cellulitis. I Basic Principles of Medical Microbiologie and Immunology F Boehringer Ingelheim International GmbH Dr. Kayser & Infectious diseases are caused by subcellular infectious entities (prions, viruses), prokaryotic bacteria, eukaryotic fungi and protozoans, metazoan animals, such as parasitic worms (helminths), and some arthropods. Definitive proof that one of these factors is the cause of a given infection is demonstrated by fulfillment of the three Henle-Koch postulates. For technical reasons, a number of infections cannot fulfill the postulates in their strictest sense as formulated by R. The History of Infectious Diseases the Past Infectious diseases have been known for thousands of years, although accurate information on their etiology has only been available for about a century. In the medical teachings of Hippocrates, the cause of infections occurring frequently in a certain locality or during a certain period (epidemics) was sought in "changes" in the air according to the theory of miasmas. This concept, still reflected in terms such as "swamp fever" or "malaria," was the predominant academic opinion until the end of the 19th century, despite the fact that the Dutch cloth merchant A. At the time, general acceptance of the notion of "spontaneous generation"-creation of life from dead organic material-stood in the way of implicating the bacteria found in the corpses of infection victims as the cause of the deadly diseases. It was not until Pasteur disproved the doctrine of spontaneous generation in the second half of the 19th century that a new way of thinking became possible. By the end of that century, microorganisms had been identified as the causal agents in many familiar diseases by applying the Henle-Koch postulates formulated by R. However, the fact that these conditions are not met does not necessarily exclude a contribution to disease etiology by a pathogen found in context. In particular, many infections caused by subcellular entities do not fulfill the postulates in their classic form. The Present the frequency and deadliness of infectious diseases throughout thousands of years of human history have kept them at the focus of medical science. The development of effective preventive and therapeutic measures in recent decades has diminished, and sometimes eliminated entirely, the grim epidemics of smallpox, plague, spotted fever, diphtheria, and other such contagions. As a result of these developments, the attention of medical researchers was diverted to other fields: it seemed we had tamed the infectious diseases. Previously unknown pathogens causing new diseases are being found and familiar organisms have demonstrated an ability to evolve new forms and reassert themselves. The origins of this reversal are many and complex: human behavior has changed, particularly in terms of mobility and nutrition. Further contributory factors were the introduction of invasive and aggressive medical therapies, neglect of established methods of infection control and, of course, the ability of pathogens to make full use of their specific genetic variability to adapt to changing conditions. The upshot is that physicians in particular, as well as other medical professionals and staff, urgently require a basic knowledge of the pathogens involved and the genesis of infectious diseases if they are to respond effectively to this dynamism in the field of infectiology. Prokaryotic and Eukaryotic Microorganisms According to a proposal by Woese that has been gaining general acceptance in recent years, the world of living things is classified in the three domains bacteria, archaea, and eucarya. This domain includes the kingdom of the heterotrophic eubacteria and includes all human pathogen bacteria. The other kingdoms, for instance that of the photosynthetic cyanobacteria, are not pathogenic. It is estimated that bacterial species on Earth number in the hundreds of thousands, of which only about 5500 have been discovered and described in detail. This domain includes forms that live under extreme environmental conditions, including thermophilic, hyperthermophilic, halophilic, and methanogenic microorganisms. The earlier term for the archaea was archaebacteria (ancient bacteria), and they are indeed a kind of living fossil. Thermophilic archaea thrive mainly in warm, moist biotopes such as the hot springs at the top of geothermal vents. The hyperthermophilic archaea, a more recent discovery, live near deep-sea volcanic plumes at temperatures exceeding 100 8C. The plant and animal kingdoms (animales and plantales) are all eukaryotic life forms. These organisms are obligate intracellular parasites that are able to reproduce in certain human cells only and are found in two stages: the infectious, nonreproductive particles called elementary bodies (0. These organisms are obligate intracellular parasites, rod- shaped to coccoid, that reproduce by binary transverse fission. They are found in a wide variety of forms, the most common being the coccoid cell (0. Fungi (Mycophyta) are nonmotile eukaryotes with rigid cell walls and a classic cell nucleus. They contain no photosynthetic pigments and are carbon heterotrophic, that is, they utilize various organic nutrient substrates (in contrast to carbon autotrophic plants). Of more than 50 000 fungal species, only about 300 are known to be human pathogens. Protozoa are microorganisms in various sizes and forms that may be free-living or parasitic. Many parasitic protozoa are transmitted by arthropods, whereby multiplication and transformation into the infectious stage take place in the vector. Medically significant groups include the trematodes (flukes or flatworms), cestodes (tapeworms), and nematodes (roundworms). These animals are characterized by an external chitin skele- ton, segmented bodies, jointed legs, special mouthparts, and other specific features. Their role as direct causative agents of diseases is a minor one (mites, for instance, cause scabies) as compared to their role as vectors transmitting viruses, bacteria, protozoa, and helminths.
Thus anxiety coping skills effective 15 mg aripiprazolum, either enteric-coated tablets or esterified forms of the antibiotic are administered anxiety tremors aripiprazolum 10mg low cost. Clarithromycin anxiety 12 year old daughter generic aripiprazolum 10 mg with visa, azithromycin depression nos definition purchase 20 mg aripiprazolum overnight delivery, and telithromycin are stable to stomach acid and are readily absorbed. Food interferes with the absorption of erythromycin and azithromycin but can increase that of clarithromycin. Azithromycin is available for intravenous infusion, but intravenous administration of erythromycin is associated with a high incidence of thrombophlebitis. It is one of the few antibiotics that diffuses into prostatic fluid, and it has the unique characteristic of accumulating in macrophages. Similarly, clarithromycin, azithromycin, and telithromycin are widely distributed in the tissues. Serum levels of azithromycin are low; the drug is concentrated in neutrophils, macrophages, and fibroblasts. Azithromycin has the longest half-life and largest volume of distribution of the four drugs (Figure 32. Fate: Erythromycin and telithromycin are extensively metabolized and are known to inhibit the oxidation of a number of drugs through their interaction with the cytochrome P450 system (see p. Interference with the metabolism of drugs such as theophylline and carbamazepine has been reported for clarithromycin (see Figure 32. Clarithromycin is oxidized to the 14-hydroxy derivative, which retains antibiotic activity. Excretion: Erythromycin and azithromycin are primarily concentrated and excreted in an active form in the bile (see Figure 32. In contrast, clarithromycin and its metabolites are eliminated by the kidney as well as the liver, and it is recommended that the dosage of this drug be adjusted in patients with compromised renal function. Epigastric distress: this side effect is common and can lead to poor patient compliance for erythromycin. Clarithromycin and azithromycin seem to be better tolerated by the patient, but gastrointestinal problems are their most common side effects (Figure 32. Cholestatic jaundice: this side effect occurs especially with the estolate form of erythromycin, presumably as the result of a hypersensitivity reaction to the estolate form (the lauryl salt of the propionyl ester of erythromycin). Ototoxicity: Transient deafness has been associated with erythromycin, especially at high dosages. Similarly, patients who are renally compromised should be given telithromycin with caution. Interactions: Erythromycin, telithromycin, and clarithromycin inhibit the hepatic metabolism of a number of drugs, which can lead to toxic accumulations of these compounds (Figure 32. In this case, the antibiotic eliminates a species of intestinal flora that ordinarily inactivates digoxin, thus leading to greater reabsorption of the drug from the enterohepatic circulation. However, because of its toxicity, its use is restricted to life-threatening infections for which no alternatives exist. Mechanism of action the drug binds to the bacterial 50S ribosomal subunit and inhibits protein synthesis at the peptidyl transferase reaction (Figure 32. Because of the similarity of mammalian mitochondrial ribosomes to those of bacteria, protein synthesis in these organelles may be inhibited at high circulating chloramphenicol levels, producing bone marrow toxicity. Antimicrobial spectrum Chloramphenicol, a broad-spectrum antibiotic, is active not only against bacteria but also against other microorganisms, such as rickettsiae. The drug is either bactericidal or (more commonly) bacteriostatic, depending on the organism. Resistance Resistance is conferred by the presence of an R factor that codes for an acetyl coenzyme A transferase. Another mechanism for resistance is associated with an inability of the antibiotic to penetrate the organism. Pharmacokinetics Chloramphenicol may be administered either intravenously or orally (Figure 32. It is completely absorbed via the oral route because of its lipophilic nature, and is widely distributed throughout the body. Excretion of the drug depends on its conversion in the liver to a glucuronide, which is then secreted by the renal tubule. Only about 10 percent of the parent compound is excreted by glomerular filtration. Adverse effects the clinical use of chloramphenicol is limited to life-threatening infections because of the serious adverse effects associated with its administration. In addition to gastrointestinal upsets, overgrowth of Candida albicans may appear on mucous membranes. Anemias: Hemolytic anemia occurs in patients with low levels of glucose 6-phosphate dehydrogenase. Other types of anemia occurring as a side effect of chloramphenicol include reversible anemia, which is apparently dose-related and occurs concomitantly with therapy, and aplastic anemia, which although rare is idiosyncratic and usually fatal. Gray baby syndrome: this adverse effect occurs in neonates if the dosage regimen of chloramphenicol is not properly adjusted. Neonates have a low capacity to glucuronylate the antibiotic, and they have underdeveloped renal function. Therefore, neonates have a decreased ability to excrete the drug, which accumulates to levels that interfere with the function of mitochondrial ribosomes. Adults who have received very high doses of the drug can also exhibit this toxicity. Interactions: Chloramphenicol is able to inhibit some of the hepatic mixed-function oxidases and, thus, blocks the metabolism of such drugs as warfarin, phenytoin, tolbutamide, and chlorpropamide, thereby elevating their concentrations and potentiating their effects (Figure 32. Clindamycin is employed primarily in the treatment of infections caused by anaerobic bacteria, such as Bacteroides fragilis, which often causes abdominal infections associated with trauma. However, it is also significantly active against nonenterococcal, gram-positive cocci. Resistance mechanisms are the same as those for erythromycin, and crossresistance has been described. Adequate levels of clindamycin are not achieved in the brain, even when meninges are inflamed. The drug is excreted into the bile or urine by glomerular filtration, but therapeutically effective levels of the parent drug are not achieved in the urine (Figure 32. Accumulation has been reported in patients with either severely compromised renal function or hepatic failure. In addition to skin rashes, the most serious adverse effect is potentially fatal pseudomembranous colitis caused by overgrowth of C. Oral administration of either metronidazole or vancomycin is usually effective in controlling this serious problem. Mechanism of action Each component of this combination drug binds to a separate site on the 50S bacterial ribosome, forming a stable ternary complex. In some cases, the enzymatic modification can change the action from bactericidal to bacteriostatic. Antibacterial spectrum the combination drug is active primarily against gram-positive cocci, including those resistant to other antibiotics (for example, methicillin-resistant staphylococci). Pharmacokinetics Quinupristin/dalfopristin is injected intravenously in a 5 percent dextrose solution (the drug is incompatible with a saline medium). The products are less active than the parent in the case of quinupristin and are equally active in the case of dalfopristin. Most of the parent drugs and metabolites are cleared through the liver and eliminated via the bile into the feces (Figure 32. Venous irritation: this commonly occurs when quinupristin/dalfopristin is administered through a peripheral rather than a central line. Arthralgia and myalgia: these have been reported when higher levels of the drugs are employed.
References