




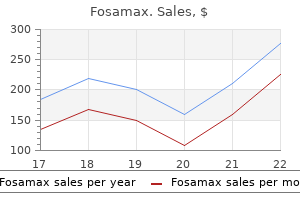
|
STUDENT DIGITAL NEWSLETTER ALAGAPPA INSTITUTIONS |

|
Michael P. Nageotte, MD
Binding of platelets to activated receptor leads to initiation of platelets activation menstruation 3 days late safe 70mg fosamax. This results in the formation of platelet aggregation which inturn causes stoppage of bleeding from ruptured blood vessel menstrual induced migraines discount 70 mg fosamax. Blood Clotting It is the one of the essential biological process divised by nature to prevent bleeding or leakage of blood from injured (damaged) blood vessels pregnancy week by week calendar discount 70 mg fosamax fast delivery. Damage to blood vessel may occur due to infections breast cancer license plate discount fosamax 70mg with mastercard, diseases, ageing, cuts, surgery etc. It involves initial formation of platelet plug to arrest bleeding and fibrin clot formation later which covers injured area and stop any further leakage of blood. Clot formation is brought about by several clotting factors present in blood (Table 32. It is so named because a factor which is not present in circulating blood is required for blood clotting. It has molecular weight of 750 kilodaltons and contains gla residues which acts as calcium binding sites. Prothrombin is 722 Medical Biochemistry activated to thrombin by factor Va-Factor Xa (prothrombinase) complex by proteolytic cleavage with elimination of gla residues. Anticoagulants like anti thrombin heparin and hirudin inhibits action of thrombin. It is another globular protein with molecular weight of 340 kilodaltons that is present in circulating plasma. Short segments of free N-terminal regions projects outwards where subunits are joined. These segments are highly negatively charged due to presence of large number of aspartate and glutamate residues. Thus charge to charge repulsion of fibrinogen molecules prevents their aggregation. At site of blood vessel injury anionic sites of membrane phospholipids are exposed. Prothrombin also binds to exposed anionic sites which is facilitated by gla residues through their interaction with Ca2+. This enzyme catalyzes formation of iso peptide bond between amide group of glutamine of one fibrin molecule and -amino group of lysine of another Biochemistry of Blood 723 fibrin molecule. Blood clotting regulation Activities of blood clotting factors are carefully controlled to prevent unwanted clot formation as well as to stop blood clotting that has been initiated. Proteinacious Proteinase inhibitors present in blood inactivates active proteinases of blood clotting to control clot formation. It is a major proteinacious serine proteinase inhibitor (serpin) that inhibits several proteinases of blood clotting. Plasminogen has high affinity towards fibrin clots and forms a complex with fibrin molecules of clot. It binds to plasminogen of plasminogen fibrin complex and activates plasminogen to plasmin by peptide bond cleavege. They are used as thrombolytic agents or clot busters in the treatment of myocardial infarction. They restore blood flow to affected cardiac tissue by dissolving clot formed in coronary artery. It prevents platelet aggregation by blocking production of thromboxane A2 and prostacyclin. Even though humans and other vertebrates have well developed mechanisms for prevention of blood loss the blood feeding insects evolved highly potent methods to bypass the host coagulation (hemostasis). Saliva of these insects contains anti coagulants like thrombin inhibitors and factor Xa inhibitors which target blood clotting factors thrombin and factor Xa or both. The blood feeding insects are responsible for the transmission of several diseases like malaria, Kyasanur forest disease in India, some forms of encephalitis in Afro-Asian countries and Lyme disease in U. Other organic compounds or constituents of Blood They are non protein nitrogenous compounds, carbohydrates, lipids, amino acids, porphyrins, bilirubin, organic acids, vitamins and hormones. Non protein nitrogenous Substances Urea, uric acid and creatinine are non protein nitrogenous substances present in blood. Likewise plasma amino acids, porphyrins and bilirubin details are given in chatper - 12 and chapter - 22 respectively. Carbohydrates Monosaccharides like glucose, fructose and sugar acids are present in blood. Plasma free fatty acids level is more in diabetes, starvation, von Gierkes disease and on high fat diet. Vitamins Normal blood contains fat soluble as well as water soluble vitamins in very small quantities. Hormones Hormones of adrenal medulla, adrenal cortex, testes, ovaries and thyroid hormones are non protein hormones present in blood. They are epinephrine, norepinephrine, glucocorticoids, mineralocorticoids, estradiol, progesterone and thyroxine. The level of these hormones are increased or decreased due to hyper or hypoactivity of glands that are involved in their production. Crystal structures of human urokinase plasminogen activator receptor bound to an antagonist peptide. These are laboratory tests done to assess function of specific organ of human body. If these tests are performed to assess function of liver then they are named as liver function tests. Function of an organ is altered due to infections, toxins, genetic factors, altered immunity, cancer or neoplasms etc. Number of tests to be performed to assess function of an organ depends on the functional roles of that organ and pathological conditions. In the case of organs having multiple functions a single test may not be adequate to assess functional integrity. Further more a change that occurs in one functional test may not be observed in another functional test. Apart from providing an insight into dysfunction of an organ these tests are useful in detection, diagnosis and prognosis of diseases affecting specific organ. Blood and urine of subject under investigation are generally used for these tests. Some of these tests are even part of routinely done investigations of a clinical biochemistry laboratory. These tests are useful in evaluating degree of dysfunction (severity), classification of diseases and directing further management of illness. Liver is involved in secretion or excretion of several components like bilirubin and bile acids. Liver function tests based on each of above functions are done routinely in laboratory. In addition measurement of serum enzymes specific to liver is helpful in assessing liver damage (Chapter 4). So measurement of bilirubin in serum and urine and serum bile acids is helpful in assessing liver damge. Elevated levels of unconjugated bilirubin occurs in prehepatic jaundice because liver cells are unable to process excess bilirubin formed. Conjugated bilirubin level raises in post hepatic jaundice because of obstruction to flow of secreted bilirubin. Both conjugated and unconjugated bilirubin levels are elevated in hepato cellular damage that occurs in hepatitis or hepatic jaundice. By combining these tests different types of jaundice can be easily differentiated. Elevated serum bile acid level suggests hepato cellular diseases like hepatitis, cirrhosis and obstruction of portal system. Serum bile acid concentration is more due to impaired uptake or secretion by hepatocytes. Tests based on excretion of xenobiotics or Clearance Tests Liver clears several xenobiotics rapidly from blood stream. Therefore elimination of these xenobiotics from the blood stream depends on functions of liver. Liver takes up these molecules by active transport mechanism involving a carrier molecule and excretes later in bile. Liver clears xenobiotics either as such or its conjugates or both and hence they are used to study liver function.
Splitting of six carbon fructose-1 42 menstrual cycle cheap fosamax 70 mg free shipping, 6-bis phosphate to 2 triose molecules is the fourth reaction of glycolysis menstrual vs pregnancy cramps purchase 70mg fosamax amex. By the action of aldolase A pregnancy 5 months discount 70 mg fosamax fast delivery, fructose-1 menstrual tent fosamax 70mg sale, 6-bisphosphate is cleaved into glyceraldehyde-3-phosphate and dihydroxy acetone phosphate. Hence, glucose-6-phosphate is converted to fructose-6-phosphate in the second reaction of glycolysis. Another important aspect of second reaction of glycolysis is related to utilization of pentoses. Pentoses are converted to fructose-6-phosphate by pentose phosphate pathway as we shall see it later. Triose phosphate isomerase catalyzes conversion of dihydroxy acetone phosphate (ketose) to glyceradehyde-3phosphate (aldose). Thus, two molecules of glyceraldehyde-3-phosphate are generated from one molecule of glucose and two high energy bonds are consumed. The enzyme catalyzes oxidation and phosphorylation of substrate to yield high energy product. Reaction 8 involves the transfer of phosphate of 3-phosphoglycerate from carbon-3 to carbon-2 of glycerate. High energy phosphate bond generation once again is the ninth reaction of glycolysis. It is catalyzed by enolase a Mn2+ or Mg 2+ dependent enzyme and reaction is reversible. Removal of one molecule of water from 3-phosphoglycerate by the enzyme converts it to phosphoenolpyruvate a high energy compound. Anaerobic glycolysis meets energy requirement of rapidly contracting skeletal muscle. Since heart is mainly aerobic organ, myocardial ischemia decreases glycolytic ability of cardiac muscle. Deficiency of enzymes of erythrocyte glycolysis (pyruvate kinase) causes haemolytic anemia. Deficiency of muscle phosphofructo kinase causes decreased muscular performance and fatigue. For example, pyruvate is converted to alanine by transamination and dihydroxy acetone phosphate serves as precursor for triglyceride formation. Two glycolytic intermediates pyruvate and glyceraldehydes-3-phosphate are used for the synthesis of cholesterol, thiamine and pyridoxine in tuberculosis, malaria and gastritis causing organisms. Regulation of Glycolysis Usually metabolic pathways are regulated by altering activities of few enzymes of that pathway. Hexokinase phosphofructo kinase and pyruvate kinase are regulatory enzymes of glycolysis. Further glucokinase, phosphofructokinase-1 and pyruvate kinase are under hormonal control also. Allosteric regulation of glycolysis Phosphofructokinase-1 is the major regulatory enzymes of glycolysis. Hormonal regulation of glycolysis Insulin increases rate of glycolysis by increasing concentration of glucokinase, phosphofructokinase-1 and pyruvate kinase. Iodoacetate, arsenate and heavy metals like Hg 2+, Ag + inhibits activity of glyceraldehyde-3-phosphate dehydrogenase. If fluoride is not added to blood the glucose concentration in the blood decreases due to consumption of glucose by erythrocytes. Because of this energy yielding reaction catalyzed by phosphoglycerate kinase is bypassed. The formation of 2, 3-bis phosphoglycerate from 1, 3-bisphosphoglycerate is catalyzed by bisphosphoglycerate mutase. Fate of Pyruvate Under aerobic conditions, pyruvate is converted to acetyl-CoA in all tissues containing mitochondria. Entry of Pyruvate into Mitochondria the mitochondrial membrane is not permeable to pyruvate, which is formed in cytosol. A specific carrier present in mitochondrial membrane transports pyruvate across mitochondrial membrane. Fate of Pyruvate in Mitochondria In mitochondria, pyruvate undergoes oxidative decarboxylation and remaining two carbon fragment is converted to acetyl-CoA. It contains lipoic acid as prosthetic group, lipoic acid is attached to amino group of lysyl residue of enzyme molecule through an amide linkage. Further shifting of acetyl moiety of acetyl lipoamide to CoA results in the formation of acetylCoA and reduced lipoamide. Regulation of Pyruvate Dehydrogenase Pyruvate dehydrogenase activity is regulated by 1. Phosphorylation and dephosphorylation of this enzyme is under hormonal control Insulin increases its activity by favouring dephosphorylation. Pyruvate dehydrogenase serve as a link between aerobic glycolysis and citric acid cycle. Since the reaction catalyzed by this enzyme is irreversible, acetyl -CoA can not be converted to pyruvate. Lactic acidemia occurs in some individuals due to deficiency of pyruvate dehydrogenase. Fate of Acetyl-CoA Under aerobic conditions or in the tissues containing mitochondria acetyl-CoA formed from pyruvate or other substances like fats and amino acids is oxidized by citric acid cycle. Two molecules of acetyl-CoAs formed from two molecules of pyruvate are oxidized by this cycle one after the other. This cyclic process starts with oxaloacetate and completes with regeneration of oxaloacetate. Carbohydrate Metabolism 165 Reaction Sequence of Citric Acid Cycle There are total eight reactions in this cycle. In the initial reaction, two carbon acetyl-CoA condenses with 4-carbon oxaloacetate to form 6 carbon citrate. Citrate formation involves carbon to carbon bond formation between methyl carbon of acetyl-CoA and carbonyl carbon of oxaloacetate. Hence, in reaction-2 it is isomerized to isocitrate a secondary alcohol and can be oxidized easily. In reaction-3, isocitrate is converted to -ketoglutarate by isocitrate dehydrogenase. In reaction-4, -ketoglutarate undergoes oxidative decarboxylation catalyzed by -ketoglutarate dehydrogenase multi enzyme complex to succinyl-CoA. In this reaction, high energy phosphate bond is generated by the action of succinylCoA synthetase on succinyl-CoA. In reaction-6, succinate is dehydrogenated by a membrane bound flavo protein succinate dehydrogenase to fumarate. It is the only enzyme of citric acid cycle, which is bound to inner mitocondrial membrane. It catalyzes the addition of water across the double bond of fumarate to give malate. The citric acid cycle is completed with the regeneration of oxalo acetate from malate in the final reaction catalyzed by malate dehydrogenase. Thus citric acid cycle operates continuously till all the acetyl-CoAs are oxidized. Energetics of Citric Acid Cycle Oxidation of acetyl-CoA in citric acid cycle is expressed as single equation below. It is the final common metabolic pathway for oxidation of carbohydrates, fats and proteins. In some liver diseases, like hepatitis and cirrhosis amphibolic role of citric acid cycle is affected. Regulation of Citric Acid Cycle Enzymes of citric acid cycle are under allosteric control. Citrate synthase, isocitrate dehydrogenase and -ketoglutarate dehydrogenase are involved in the regulation of citric acid cycle and their activities are allosterically regulated. Further Ca 2+ seems to increase the activities of isocitrate dehydrogenase and -ketoglutarate dehydrogenase. As such it is not toxic, but in the body, it is converted to fluoroacetyl-CoA, which gives rise to fluorocitrate after condensing with oxaloacetate. The conversion of non-toxic compound to toxic compound inside the body is called as lethal synthesis. Energetics of Aerobic Oxidation of Glucose Under aerobic conditions, complete oxidation of glucose occurs in three stages.
Coupled oxidation-reduction reactions involving transfer of electrons or hydrogen atoms from one compound to another compound also occurs in various metabolisms women's health clinic lubbock quality 35mg fosamax. Specific enzymes menstrual like cramping in third trimester discount 35mg fosamax otc, coenzymes are involved in the electron transfer reactions in living systems menstruation 10 days delayed discount fosamax 35 mg without prescription. Several important biological oxidation reactions are directly associated with respiratory O2 breast cancer rash fosamax 70 mg with visa. Apart form respiratory chain, several enzymes use O2 as final electron acceptor and produce H 2 0 2. Several new compounds are synthesized by directly incorporating O 2 into certain substances. Respiratory O 2 is also required for the removal of toxins and drugs from the body. Biological oxidation provides means for the regeneration of coenzymes, which are used in metabolism. Transfer of electrons is impaired in certain disease like encephalopathy, lactic acidosis and mitochondrial myopathy. As a result of this, energy production in cardiac cells is blocked, which lead to necrosis. In some instances like high altitudes, surgeries to maintain normal functioning of body or cells O2 supply is essential. Though O2 is essential for survival of cells at high concentration it is toxic to cells. Many enzymes, coenzymes and several carrier molecules are involved in oxidation-reduction (electron transfer) reactions of biological system. They are dehydrogenases, oxidases, oxygenases, hydroperoxidases, cytochromes, ubiquinone and iron-sulfur proteins. Dehydrogenases the dehydrogenases are divided into two groups based on the coenzyme (prosthetic group) they require for activity. Nicotinamide-dependent dehydrogenases They catalyze the transfer of hydrogen (electrons) from one substrate to another substrate in a coupled oxidation-reduction reaction. The coenzymes are reduced by a substrate of dehydrogenase and reoxidized by an hydrogen acceptor catalyzed by another dehydrogenase. Since these enzymes can not use oxygen as hydrogen acceptor they may be called as anaerobic dehydrogenases. From the pair of hydrogens, an hydride ion (H-) having two electrons is attached to nicotinamide and remaining hydrogen is released as free proton (H+). Likewise reduction of substrates by these enzymes involves transfer of hydrogens from nicotinamide. Riboflavin-dependent dehydrogenases They catalyze the removal of hydrogen from substrates. Since oxygen is not (electron) hydrogen acceptor these are referred as riboflavin dependent anaerobic dehydrogenases (Figure 11. Hence, these can be referred as a riboflavin dependent aerobic dehydrogenases (Figure 11. Oxidation of a substrate involves reduction of isoalloxazine ring via semiquinone. Cytochrome a 3 (4Fe2+) + O2 + 4H+ Cytocrome a3 (4Fe3+) + 2H2O Out of the two subunits only cytochrome a3 can directly react with oxygen. Cytochrome oxidase catalyzes the transfer of electrons from cytochrome c to molecular oxygen. Oxygenases They catalyze incorporation of oxygen directly into substrate molecules. Tryptophan dioxygenase (b) Mono oxygenases They catalyze incorporation of one atom of oxygen into substrate. These enzymes are loosely referred as hydroxylases and (or) mixed function oxidases. Cytochrome P450 hydroxylases Hydroperoxidases these enzymes catalyze breakdown of H2O2 which is produced in the body during reduction of oxygen to water. Cytochrome b and c1 are integral membrane proteins and they are constituents of cytochrome reductase complex. Cytochromes other than the components of respiratory chain are Cytochrome P450 It is so named because its complex with carbon monoxide absorbs light at 450 nm. Cyt P450-hydroxylase is responsible for the formation of carcinogens from pre-carcinogens present in food. Mitochondrial cytochrome P450 hydroxylase It is present in mitochondria of liver, adrenal cortex, testes, ovaries and kidneys. In adrenal cortex cyt P450 hydroxylase is responsible for hydroxylation of steroid hormones. It is responsible for hydroxylation of bile acids in liver, steroid hormones in testes and ovaries. It participates in coupled oxidation reduction reactions of respiratory chain via semiquinone intermediate (Figure 11. Biological Oxidation and Respiratory Chain 271 Free energy It is the potential energy of a substance. Free energy of a substance is represented by G (Gibbs) and it is difficult to measure G directly. In any chemical reaction the free energy content of reactant and product are not same. Under such conditions, the conversion of A to B is accompanied by release of free energy and reaction occurs with free energy decrease. Hence, the conversion of A to B takes place when energy is supplied and reaction occurs with free energy increase. Determination of G the free energy change of a chemical reaction A B is determined by equation. High energy compounds the hydrolysis of these compounds is accompanied by release of large amount of free energy. The energy released when an high energy compound is hydrolyzed is not due to bond that is hydrolyzed. The electronic structure of these compounds is responsible for the release of large free energy on hydrolysis. Thioesters They are formed from the condensation of coenzyme A, a thiol with carboxylic acids. For example, hydrolysis of acetyl-CoA to acetic acid and water is accomanied by release of 7. The two acids involved in the mixed anhydride formation are carboxylic acid and phosphoric acid. The oxidant (acceptor) and reductant (donor) of a redox reaction are known as redox pair or redox couple. Likewise, if E01 is positive for a redox pair then, it accepts electrons or undergo reduction. Electron transfer and free energy When electrons flow from electronegative redox pair towards electropositive redox pair free energy is liberated. The amount of free energy liberated when electrons move from one redox pair to another is given by the equation. G0 = -nfE1 0 G0 = standard free energy change in calories n = number of electrons transferred f = faraday (23. The equation also indicates that the amount of free energy liberated depends on difference of the redox potential between two redox pairs. Electron transport chain consist of various electron transport or electron carrier molecules. The position of a particular component in the respiratory chain depends on its redox potential. The components of respiratory chain are arranged in the order of increasing redox potential. Starting components have negative redox potential and terminal components have positive redox potential. Therefore, in the respiratory chain, electrons flow from negative to positive (Figure 11.
Benzpyrene women's health center utexas purchase 70 mg fosamax with mastercard, aminopyrine women's health issues and physical therapy purchase 70mg fosamax amex, aniline breast cancer nail decals buy fosamax 35 mg line, morphine womens health zymbiotix cheap fosamax 70 mg free shipping, and benzphetamine are hydroxylated, increasing their solubility and aiding their excretion. Many drugs such as phenobarbital have the ability to induce the synthesis of cytochromes P450. Liver microsomal cytochrome P450 hydroxylase does not require the iron-sulfur protein Fe 2 S2. Mitochondrial cytochrome P450 systems are found in steroidogenic tissues such as adrenal cortex, testis, ovary, and placenta and are concerned with the biosynthesis of steroid hormones from cholesterol (hydroxylation at C2 2 and C2 0 in side-chain cleavage and at the 11 and 18 positions). In addition, renal systems catalyzing 1 - and 24hydroxylations of 25-hydroxycholecalciferol in vitamin D metabolism-and cholesterol 7 -hydroxylase and sterol 27-hydroxylase involved in bile acid biosynthesis in the liver (Chapter 26)-are P450 enzymes. The ease with which superoxide can be formed from oxygen in tissues and the occurrence of superoxide dismutase, the enzyme responsible for its removal in all aerobic organisms (although not in obligate anaerobes) indicate that the potential toxicity of oxygen is due to its conversion to superoxide. Superoxide is formed when reduced flavins-present, for example, in xanthine oxidase-are reoxidized univalently by molecular oxygen. Superoxide can reduce oxidized cytochrome c or be removed by superoxide dismutase: In this reaction, superoxide acts as both oxidant and reductant. Thus, superoxide dismutase protects aerobic organisms against the potential deleterious effects of superoxide. The enzyme occurs in all major aerobic tissues in the mitochondria and the cytosol. Although exposure of animals to an atmosphere of 100%oxygen causes an adaptive increase in superoxide dismutase, particularly in the lungs, prolonged exposure leads to lung damage and death. Antioxidants, eg, -tocopherol (vitamin E), act as scavengers of free radicals and reduce the toxicity of oxygen (Chapter 44). Oxidoreductases have a variety of functions in metabolism; oxidases and dehydrogenases play major roles in respiration; hydroperoxidases protect the body against damage by free radicals; and oxygenases mediate the hydroxylation of drugs and steroids. Tissues are protected from oxygen toxicity caused by the superoxide free radical by the specific enzyme superoxide dismutase. Most of this takes place inside mitochondria, which have been termed the "powerhouses" of the cell. A number of drugs (eg, amobarbital) and poisons (eg, cyanide, carbon monoxide) inhibit oxidative phosphorylation, usually with fatal consequences. Several inherited defects of mitochondria involving components of the respiratory chain and oxidative phosphorylation have been reported. The outer membrane is characterized by the presence of various enzymes, including acyl-CoA synthetase and glycerolphosphate acyltransferase. Note that the enzymes of the citric acid cycle and -oxidation (Chapters 22 & 17) are contained in mitochondria, together with the respiratory chain, which collects and transports reducing equivalents, directing them to their final reaction with oxygen to form water, and the machinery for oxidative phosphorylation, the process by which the liberated free energy is trapped as high-energy phosphate. Components of the Respiratory Chain Are Contained in Four Large Protein Complexes Embedded in the Inner Mitochondrial Membrane Electrons flow through the respiratory chain through a redox span of 1. The four complexes are embedded in the inner mitochondrial membrane, but Q and cytochrome c are mobile. The Fe-S take part in single electron transfer reactions in which one Fe atom undergoes oxidoreduction between Fe2+ and Fe3+. Flow of electrons through the respiratory chain complexes, showing the entry points for reducing equivalents from important substrates. Of the eight H+ removed from the matrix, four are used to form two water molecules and four are pumped into the intermembrane space. Since the inner mitochondrial membrane is impermeable to ions in general and particularly to protons, these accumulate in the intermembrane space, creating the proton motive force predicted by the chemiosmotic theory. F1 is attached to a membrane protein complex known as F0, which also consists of several protein subunits. The enzyme complex consists of an F 0 subcomplex which is a disk of "C" protein subunits. The subunit fits inside the F1 subcomplex of three and three subunits, which are fixed to the membrane and do not rotate. For clarity, not all the subunits that have been identified are shown-eg, the "axle" also contains an subunit. Two more high-energy phosphates per mole of glucose are captured in the citric acid cycle during the conversion of succinyl CoA to succinate. These reactions are known as oxidative phosphorylation at the respiratory chain level. Under certain conditions, the concentration of inorganic phosphate can also affect the rate of functioning of the respiratory chain. The remaining free energy that is not captured as high-energy phosphate is liberated as heat. They may be classified as inhibitors of the respiratory chain, inhibitors of oxidative phosphorylation, and uncouplers of oxidative phosphorylation. Sites of inhibition of the respiratory chain by specific drugs, chemicals, and antibiotics. N - Ethylmaleimide, hydroxycinnamate, and atractyloside inhibit the indicated systems. The uncoupler that has been used most frequently is 2,4-dinitrophenol, but other compounds act in a similar manner. Thermogenin (or the uncoupling protein) is a physiological uncoupler found in brown adipose tissue that functions to generate body heat, particularly for the newborn and during hibernation in animals (Chapter 25). Such systems are necessary for uptake and output of ionized metabolites while preserving electrical and osmotic equilibrium. However, dicarboxylate and tricarboxylate anions and amino acids require specific transporter or carrier systems to facilitate their passage across the membrane. Monocarboxylic acids penetrate more readily in their undissociated and more lipid-soluble form. The net uptake of malate by the dicarboxylate transporter -Ketoglutarate transport also requires an requires inorganic phosphate for exchange in the opposite direction. The net uptake of citrate, isocitrate, or cis aconitate by the tricarboxylate transporter requires malate in exchange. It is believed that active uptake of Ca2+ by mitochondria occurs with a net charge transfer of 1 (Ca+ uniport), possibly through a Ca2+ /H+ antiport. The H+ /Pi symport Ionophores Permit Specific Cations to Penetrate Membranes Ionophores are lipophilic molecules that complex specific cations and facilitate their transport through biologic membranes, eg, valinomycin (K+). The transfer of reducing equivalents through the mitochondrial membrane requires substrate pairs, linked by suitable dehydrogenases on each side of the mitochondrial membrane. Although this shuttle is present in some tissues (eg, brain, white muscle), in others (eg, heart muscle) it is deficient. The complexity of this system is due to the impermeability of the mitochondrial membrane to oxaloacetate, which must react with glutamate to form aspartate and cytosol.
All the above phenotype characters of living organisms are intimately related to functions of proteins breast cancer team names discount fosamax 35 mg free shipping. Such long molecule is present in nucleus whose dimension is less than 5 microns (5 u) (1 u = 10-3 mm) women's health clinic redwood city 70mg fosamax mastercard. W ra pp ed D N A N u cleo so m e L in ke r D N A H isto n e (H I) N u cleo so m e L in ke r D N A (a) (b) womens health upenn discount 35mg fosamax overnight delivery. Adeno virus (cold virus) pregnancy lingerie fosamax 70mg with visa, Herpes virus and Pox virus are examples for double stranded viruses. The pyrimidine rich strand dissociates from complementary strand and folds back on itself to lie in the major groove and hydrogen bonded to purine rich strand. Intrastrand base paring among complementary bases allows folding of liner molecule. They are methylated adenine, guanine, cytosine and thymine, dihydrouracil, pseudo uridine, isopentenyl adenine etc. Intra strand base pairing between complementary base generates double helical segments or loops. Resistant to hydrolysis by alkali because of absence of hydroxyl group on 2 carbon atom of deoxyribose 6. Some serve as anti-sense molecules and interfere with transcription and translation. They are also involved in genomic imprinting, X-chromosome in activation, germ cell formation, Meiosis, oxidative stress and diseases like cancer. Completion of human genome project is an extra ordinary achievement of man comparable to that of landing on moon. Sequencing is done by specially designed high-speed sequencers with little human involvement, which have very high (through) put. Though both groups used dideoxy method for sequencing, they adopted different approach for sequencing. Human genome consortium adopted Top-down approach in which genome is first segregated into smaller segments in a stepwise manner and when pieces are small enough, they are sequenced. After sequencing, these individual pieces are joined together to get chromosome of their origin by back tracking. Shot-gun procedure or Bottom-up approach is adopted by Celera genomics headed by Venter for sequencing. All the sequenced fragments are assembled by matching identifying pairs of sequences among any two fragments. Nucleic Acids 415 When sequencing of fragments is completed, its genes or protein coding regions are detected by using computational biology procedures. It is genes-containing array of sequences that determines coordination, communication and functions of the cells which are ultimately responsible for proper health and well being of an individual. Human genome sequence provide some solutions to atleast few medical problems which remained mystery. Sequencing of human genome allows mapping of disease genes on specific locations on chromosomes. Polymorphic severe combined immuno deficiency Duchenne muscular dystrophy, Adreno leukodystrophy Prostate cancer, Adenocarcinoma Identification of new disease genes may provide starting point for the development of new diagnostic kits. Further genome sequence enables identification culprit genes involved in diseases whose underlying causes are yet to be elucidated. Sequence information provides molecular details of signal transduction, differential expression of gene products in various tissues during normal growth, uncontrolled growth in tumour tissues. Synthetic genes or synthetic nucleotide fragments are generated by automated solid phase chemical synthesis. It replicates in laboratory, which is one of the most important property of any life form. Several other new life forms will be generated soon and the consequences of these attempts by man may be beneficial to human race. Crothers, Nucleic acids: structure, properties and functions, University Science Books, 2000. This genetic information flow is popularly called as central dogma of molecular genetics. Therefore these two processes are also included in central dogma in 1970 (Figure 17. During this period, concentration of deoxy ribo nucleotides increases to several folds. When cell divides, each daughter cell must contain entire genetic information of parent cell. They catalyze polymerization of deoxyribonucleotides into nucleic acids or polynucleotides. After the formation of initial phosphodiester bond, the primer is elongated by further addition of deoxy ribonucleotides. But observed time for replication was 4-8 hours in eukaryotes and less than two hours in prokaryotes. Coli starts at a unique origin, proceeds in opposite directions simultaneously and completed in 100 minutes. Regulation of replication Replication must occur only when cell prepares for division. However, the methylation of nucleotides of ori C may be one way of controlling replication. Medical importance Several compounds block replication by acting at various stages of replication. By blocking replication these compounds slows the division of rapidly growing cancer cells, bacterial cells and viruses. Inhibitors of replication are used as anti-cancer agents, anti-bacterial or anti-biotics and anti-viral agents. Acyclovir It is another nucleoside analog used in viral infection caused by herpes virus. It is an analog of guanosine in which pentose is replaced with three carbon sugar. Replication origins and cancer Large number of replication origins in eukaryotes are puzzling molecular biologists for long time. During G1 phase of cell cycle replication origins are prepared to fire and subsequently activated in S-phase. Therefore, reduction in replication origins alters progression of S-phase, mitosis and chromosomal translocations. Since gross chromosomal rearrangements are associated with cancer development, large number of replication origins are necessary for normal cell division. Thus, large number of replication origins prevents a normal cell turning into cancer cell. In future diagnostic tests that can predict a cell to become cancer cell based on replication origins may be developed. Leishmaniass and trypanosomiasis caused by protozoan parasites leishmania donovani and trypanosoma brucei affects millions of people worldwide. Some examples of topoisomerase inhibitors with potent anti-trypanosomal activity are pentamidine, berenil, samorine etc. Xeroderma pigmentosum this disease is due to deficiency of endonuclease involved in excision repair. Other symptoms are thorny growth of skin, corneal ulceration, scarred eye lids etc. More than one polymerase can transcribe same template at different places simultaneously. The transcription errors are tolerable because of formation of large number of copies. Aflatoxin A fungus that grows on moist ground nut produces aflatoxin, which inhibit transcription.
Discount fosamax 35mg on-line. Types of MOM's During EXAMS .. | #Students #Sketch #Roleplay #Anaysa #ShrutiArjunAnand.