


|
STUDENT DIGITAL NEWSLETTER ALAGAPPA INSTITUTIONS |

|
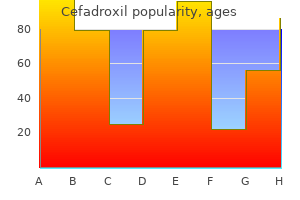
"Best 250mg cefadroxil, antibiotic home remedies".
T. Mojok, M.B. B.CH., M.B.B.Ch., Ph.D.
Clinical Director, San Juan Bautista School of Medicine
Thyroxin is transported in the blood plasma complexed to a binding protein called thyroxine binding globulin antibiotic ointment for burns buy 250 mg cefadroxil otc. Triiodothyronine treatment for dogs bad breath order 250 mg cefadroxil otc, which hormonally is the more potent of the two bacteria define trusted 250 mg cefadroxil, but is not as abundant antibiotic 101 purchase cefadroxil 250mg fast delivery, is not as firmly bound to the binding protein. Most of the secreted thyroid hormone (90%) is thyroxine but is converted to the more active form, triiodothyronine, by peripheral target tissues. The kidney and liver are important deiodinators of thyroxin and convert it to the functionally more potent triiodothyronine. Triiodothyronine then bids to a nuclear receptor in cells of the target organ the net result of which is an increase in oxygen consumption and metabolic rate. Thyroid hormone has general effects on the metabolic rate of most tissues, and among its functions are increased carbohydrate metabolism, increased rate of intestinal absorption, increased kidney function, increased heart rate, increased ventilation, normal body growth and development, and increased mental activities. The thyroid also contains a smaller number of cells variously called parafollicular, light, or C cells, which are present adjacent to the follicular epithelium and in the delicate connective tissue between follicles. The C cells adjacent to the follicular epithelium appear to be sandwiched between the bases of follicular cells and lie immediately adjacent to the basal lamina; parafollicular cells never directly border on the lumen of the follicle. In electron micrographs, the parafollicular cells show numerous moderately dense, membrane-bound secretory granules that measure 10 to 50 nm in diameter. The cytoplasm also contains occasional profiles of granular endoplasmic reticulum, scattered mitochondria, and poorly developed Golgi complexes. Parafollicular cells secrete calcitonin (thyrocalcitonin), another polypeptide hormone that regulates blood calcium levels. Calcitonin lowers blood calcium by acting on osteocytes and osteoclasts to suppress resorption of calcium from bone and its release into the blood. Thus, calcitonin has an effect opposite that of parathyroid hormone, serves to control the action of parathyroid hormone, and helps regulate the upper levels of calcium concentration in the blood. Adrenal Gland the adrenal glands in humans are a pair of flattened, triangular structures with a combined weight of 14 to 16 gm. The adrenal gland is a complex organ consisting of a cortex and medulla, each differing in structure, function, and embryonic origin. The capsule contains a rich plexus of blood vessels - mainly small arteries - and numerous nerve fibers. Some blood vessels and nerves enter the substance of the gland in the trabeculae that extend inward from the capsule and then leave the trabeculae to enter the cortex. The parenchyma of the adrenal cortex consists of continuous cords of secretory cells that extend from the capsule to the medulla, separated by blood sinusoids. The cortex is subdivided into three layers according to the arrangement of the cells within the cords. These cortical layers consist of an outer zona glomerulosa (10%), a middle zona fasciculata (75%), and an inner zona reticularis (15%). The columnar cells are arranged into ovoid groups or arcades and have centrally placed spherical nuclei. In electron micrographs, the cells show a well-developed smooth endoplasmic reticulum and numerous mitochondria that are evenly distributed throughout the cytoplasm. Occasional lipid droplets and scattered profiles of granular endoplasmic reticulum also are present. Zona fasciculata forms the widest zone of the cortex and consists of long cords that usually are one or two cells thick. The cords run parallel to one another, separated by sinusoids that are lined by an attenuated endothelium. The cytoplasm contains numerous rounded mitochondria with tubular cristae, abundant smooth endoplasmic reticulum, and well-developed granular endoplasmic reticulum. Many lipid droplets also are present and contain neutral fat, fatty acids, and fatty acyl esters of cholesterol; these represent stored precursors for the synthesis of the steroid hormones secreted by the zona fasciculata. Zona reticularis forms the innermost zone and is made up of a network of irregular, anastomosing cords that also are separated by sinusoids. In general, the cells of this zone resemble those of zona fasciculata except that they are smaller, the cytoplasm contains fewer fat droplets, the nuclei stain more deeply, and lipofuscin granules are prominent.
Permanence is the duration of the plasticizer effect; the plasticizer should remain within the polymer film to retain its effect infection 5 weeks after abortion discount 250mg cefadroxil free shipping, so it should have a low vapor pressure and diffusion rate antibiotic drug classes discount cefadroxil 250 mg free shipping. Commonly used plasticizers include phthalate esters antimicrobial keyboard covers cefadroxil 250 mg mastercard, citrate esters virus outbreak movies order cefadroxil 250mg without prescription, triacetin, propylene glycol, polyethylene glycols, and glycerol. Film coating provides an opportunity to color tablets, and most film coats will contain pigments or opacifiers. Insoluble pigments are normally preferred to soluble dyes for a number of reasons. Solid pigments produce a more opaque coat than dyes, protecting the tablet from light. The presence of insoluble particles in the suspension allows the rate of solid application to the tablet to be increased without having an adverse effect on the viscosity of the coating suspension, improving productivity. Film coating of tablets requires both the process and the formulation to be optimized if a uniform and intact coating is to be achieved. The most common flaws, their cause, and possible remedies are described in Table 16. It has been emphasized to this point that the coat should not delay the release of the drug substance from the tablet. It is possible to design a coat that will modify the release of a drug substance for a beneficial effect. Oral Solid Dosage Forms Table 16 Film-Coating Defects Flaw Wrinkling or blistering Film detaches from tablet surface, causing blister that can burst to form wrinkles. Cause Gases forming on tablet surface during coating; exacerbated by poor adhesion of film to tablet surface. High internal stresses in film relieved by pulling the film off the Surface of the intagliation. Cracking, splitting, peeling the film cracks on the crown of the surface or splits on the tablet edge. High stresses in the film that cannot be relieved due to the strong Adhesion of the film to the tablet surface. The most common type of modified-release coating is the enteric coat, which is designed to prevent release of the drug substance in the stomach because the drug is either irritant to the gastric mucosa or it is unstable in gastric juice. The most common method of achieving an enteric coating is to apply a polymer that contains acidic substitutions, thus giving it a pHdependent solubility profile. This is normally applied to multiparticulate systems where particles will be coated with differing thicknesses of waterinsoluble polymers such as ethylcellulose. Compression Coating the third type of tablet coating used in pharmaceuticals is compression coating. This is achieved by placing the core in a large die that already contains some of the coating formulation. The three types of coating have now been described, and the relative merits of each approach are summarized in Table 18. Hard gelatin capsules are rigid two-piece capsules made from gelatin, water, and colorants. The capsules are produced as empty shells consisting of a cap and body, which during the manufacture of the finished product are separated, filled with the formulation, and then rejoined. While many of the challenges facing the formulator of tablets are still present, the need for a free-flowing material, powder homogeneity, lubricity, and optimizing the biopharmaceutical properties, the challenges of forming a robust compact have been removed. Capsules have traditionally been filled with solid formulations such as powder mixes and granulations, but increasingly, multiparticulate formulations, liquid, and semisolid fills are being developed. The filling speeds of capsule machines are lower than the speeds of the fastest tablet presses, and the cost of the capsule shells makes them a more expensive dosage form. Certain materials are unsuitable for inclusion in capsule shells due to incompatibility with gelatin, or because of their affinity for water. One advantage sometimes claimed for capsules is ease of swallowing due to their elongated shape, but this may be countered by the fact that capsules are often larger than corresponding tablets due to the reduced compression of the powder and incomplete filling of the shells. Furthermore, there are concerns that capsules are more prone to sticking in the esophagus than tablets following swallowing.
Small areas of amorphous material on the particle surface produced by particle attrition antibiotics not helping uti buy cefadroxil 250 mg fast delivery, too small to be detected by techniques such as X-ray powder diffraction bacteria scientific name discount cefadroxil 250 mg online, can have dramatic effects on the wetting characteristics antibiotic classes buy 250mg cefadroxil fast delivery. It is possible to granulate a powder simply by adding water or an organic solvent to a powder antibiotic resistance review 2015 buy cheap cefadroxil 250mg line. Provided that the liquid is able to wet the powder surface, it will form liquid bridges. When the granulate dries, the crystallization of any solids that had dissolved in the liquid will form solid bonds between the particles. These bonds will usually be fairly weak, and friable granulates will be formed; often the granules will not be sufficiently robust to withstand the drying process. It is usual, therefore, to include granulating agents or binders to granulations to increase the granule strength. Granulating agents are usually hydrophilic polymers that have cohesive properties that both aid the granulation process and impart strength on the dried granulate. For a granulating agent to be effective, it is vital that it is able to form a film on the particle surface. Rowe (1989) has suggested that binders should be selected on the basis of their spreading coefficients, where the spreading coefficient is defined as the difference between the "work of adhesion" of the binder and the substrate and the "work of cohesion" of the binder. The most common method of adding binders is as a solution in the granulating fluid. This approach, which has the advantage of not requiring a solution manufacturing step in production, is likely to result in incomplete hydration of the polymers during processing, which will affect the quality of the final granulate. The level of binder used needs to be a balance between the level required to produce a robust, compressible granulate and the biopharmaceutical properties. As the granule strength increases, there is often an adverse effect on disintegration and dissolution. To optimize the dissolution of a drug from a granulated product, it is important that the granule should disintegrate into its primary particles as rapidly as possible, and it is usual to position at least a portion of any disintegrant inside the granules. A number of processing methods are commonly used within the pharmaceutical industry. Each method will impart particular characteristics and, as such, granulates produced by each method may not be equivalent in terms of either their physical properties (which will influence subsequent performance on tablet machines) or their biopharmaceutical properties. This latter point is well recognized by regulatory authorities that regard changes in the granulation method as potentially having a significant effect on the bioavailability of poorly soluble, poorly permeable compounds. This document concludes that changing the type of granulation process used can, for some drug substances, result in the need to perform a bioequivalence study prior to the change being granted regulatory approval. The three main methods of producing pharmaceutical granulates are low-shear granulation, high-shear granulation, and fluid bed granulation. Low-shear mixers encompass machines such as Z-blade mixers and planetary mixers, which, as their names suggest, impart relatively low shear stresses onto the granulate. Widely used in the past, this approach has largely been superseded by high-shear mixers. The low level of shear applied is often insufficient to ensure good powder mixing, so a premix is often required. The process is forgiving in terms of the amount of liquid added, although it does result in long granulation times. The degree of ball growth tends to be uncontrolled because there is insufficient shear to break up the plastic agglomerates, so a wet screening stage is almost always necessary prior to drying to reduce the larger agglomerates. High-shear granulators are closed vessels that normally have two agitators; an impeller, which normally covers the diameter of the mixing vessel, and a small chopper positioned perpendicular to the impeller. The powders are dry mixed using the impeller, and then the granulating fluid is added. Wet massing takes place using the impeller and the chopper, and granulation is usually completed in a number of minutes. The granulation process can be controlled using an appropriate combination of impeller and chopper speeds and time. The ability of the chopper to limit the size of the agglomerates can negate the need for a wet screening stage for many granulates.