




|
STUDENT DIGITAL NEWSLETTER ALAGAPPA INSTITUTIONS |

|
"Order sustiva 600mg, symptoms checker".
Z. Alima, MD
Assistant Professor, Washington State University Elson S. Floyd College of Medicine
Prenatal diagnosis medicine universities sustiva 200 mg free shipping, in association with genetic counseling symptoms of breast cancer effective sustiva 600mg, allows for an informed reproductive decision if the fetus is affected treatment for hemorrhoids proven sustiva 200 mg. Methods available: the available diagnostic methods vary in sensitivity and specificity medicine 013 generic sustiva 200 mg. Visualization of the fetus, for example, by ultrasound or fiberoptic devices (fetoscopy), is useful only if the genetic abnormality results in gross anatomic defects (for example, neural tube defects). For example, the presence of high levels of fetoprotein is associated with neural tube defects. Staining and cell sorting techniques permit the rapid identification of trisomies and translocations that produce an extra chromosome or chromosomes of abnormal lengths. Early efforts to diagnose sickle cell anemia: In the past, prenatal diagnosis of hemoglobinopathies involved the determination of the amount and kinds of Hb synthesized in red cells obtained from fetal blood. However, the invasive procedures to obtain fetal blood have a high mortality rate (approximately 5%), and diagnosis cannot be carried out until late in the second trimester of pregnancy when HbS begins to be produced. Thus, the A-to-T mutation in codon 6 of the bS-globin gene eliminates a cleavage site for the enzyme. Identification of the gene: Determinining the presence of the mutant gene by identifying the polymorphism marker can be done if two conditions are satisfied. It is then possible to trace the inheritance of the gene within a family without knowledge of the nature of the genetic defect or its precise location in the genome. Early diagnosis and treatment are essential in preventing severe neurologic damage in affected individuals. It can amplify the sequence, even when the targeted sequence makes up less than one part in a million of the total initial sample. The 3 -hydroxyl end of each oligonucleotide points toward the target sequence (see Figure 33. Each strand binds a complementary primer, and the cycle of chain extension is repeated. Thus, each newly synthesized strand can act as a template for the successive cycles (see Figure 33. They can also be used to subclassify cancers, such as breast cancer, to optimize treatment. When analyzing the abundance and interactions of a large number of proteins, automated methods involving a variety of techniques, such as mass spectrometry and two-dimensional electrophoresis, are used. When investigating one, or a limited number of proteins, labeled antibodies are used to detect and quantify specific proteins and to determine posttranslational modifications. The probe used consists of an antibody specific for the particular protein to be measured. The antibody is covalently bound to an enzyme, which will produce a colored product when exposed to its substrate. The amount of color produced is proportional to the amount of antibody present and, indirectly, to the amount of protein in a test sample. Western blots: Western blots (also called immunoblots) are similar to Southern blots, except that protein molecules in the sample are separated by electrophoresis and blotted (transferred) to a membrane. The probe is a labeled antibody, which produces a band at the location of its antigen. Because these assays sometimes give false positives, however, Western blots, which are more specific, are often used as a confirmatory test (Figure 33. Proteomics the study of the proteome or all the proteins expressed by a genome, including their relative abundance, distribution, posttranslational modifications, functions, and interactions with other macromolecules, is known as proteomics. The 20,000 to 25,000 protein-coding genes of the human genome translate into well over 100,000 proteins when posttranscriptional and posttranslational modifications are considered. Although a genome remains essentially unchanged, the amounts and types of proteins in any particular cell change dramatically as genes are turned on and off. Because somatic gene therapy changes only the targeted somatic cells, the change is not passed on to the next generation. Challenges of gene therapy include development of vectors, achievement of long-lived expression, and prevention of side effects such as an immune response. It utilized mature T lymphocytes transduced ex vivo with a viral vector (Figure 33. Since 1990, only a small number of patients (with a variety of disorders, such as hemophilia, cancers, and certain types of blindness) have been treated with gene therapy, with varying degrees of success. If the gene becomes successfully integrated into a chromosome, it will be present in the germline of the resulting animal and can be passed along from generation to generation.
Diseases
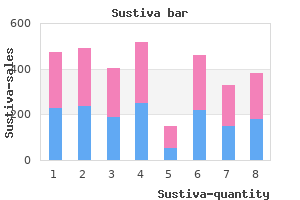
As the cost of the services offered by these companies declines and the claims regarding these services increase symptoms leukemia buy sustiva 600 mg overnight delivery, it seems reasonable to investigate what consumers may actually gain from such information treatment vaginitis purchase 600mg sustiva. Relative risk was reported to be increased by 1 company and reported to be decreased by the other company for 5 of the 14 health conditions for which both companies reported such information: colorectal cancer medicine cabinets with mirrors cheap 600 mg sustiva overnight delivery, Crohn disease symptoms throat cancer discount sustiva 600mg free shipping, heart attack, prostate cancer, and restless leg syndrome. One sample had 4 discordant results and the other sample had 2 discordant results; the discordant relative risk values are in boldface type. The insubstantial magnitude of the risk information raised doubts about clinical validity. Moreover, there were sometimes substantial differences between the 2 companies in the level of risk reported, which calls into serious question the analytic validity of the findings. A possible limitation of our findings is that these analyses were performed in 2008. Finally, the report states, Perhaps most disturbing, one company told a donor that an above average risk prediction for breast cancer meant she was "in the high risk of pretty much getting" the disease, a statement that experts found to be "horrifying" because it implies the test is diagnostic. Monica Gulisano Genetic testing is available for nearly 300 specific targeted mutations associated with various disorders [1]. Such testing is marketed directly to consumers, who can purchase it without any involvement on the part of their health care provider. Over the past decade, great advances have been made in discovering the genetic basis of monogenic diseases such as Tay-Sachs disease and cystic fibrosis, but finding meaningful associations between genetic variants and polygenic diseases such as diabetes, cancer, and cardiovascular disease is more difficult and will require more time. Although there seems to be strong public interest in testing for susceptibility to psychiatric disorders, little is known about the impact on individuals of receiving the results of such genetic tests [11]. Further contributing to the potential for confusion among consumers are claims made by companies on their Web sites and in their marketing materials. One of the presumed benefits of genetic testing is its potential to motivate lifestyle changes, although the ability of such testing to encourage healthy behavior is disputable [2]. Current research suggests that consumers believe that they will change their health behavior once they know their genetic test results. However, studies of actual changes in behavior after people receive the results of genetic testing have come to mixed conclusions. In a randomized trial of the use of personalized genetic risk counseling to motivate diabetes prevention [5], subjects were randomly assigned to receive genetic testing or no genetic testing. Those who had been tested were then ranked from highest to lowest risk, and those in the top and bottom quartiles were enrolled in a diabetes prevention program along with untested control subjects. Few significant differences were found in motivation, program attendance, and weight loss when the lowest-risk and highest-risk groups were separately compared with the control group [5]. This prompted her physician to obtain standard clinical testing, leading to a diagnosis of celiac disease in both the patient and her daughter. Such claims conflate marginally elevated risk assessment with diagnostic testing, the former being no substitute for appropriate clinical assessment and diagnostic evaluation. Critics have worried that the confusion created by complicated risk profiles in the absence of proper genetic counseling may provoke unnecessary fear and worry in consumers. This may be because consumers who purchase such tests tend to have high educational levels and knowledge of genetics [2]. Some companies claim to offer a genetically tailored diet plan and nutritional supplement recommendations that will protect against the diseases to which an individual is genetically predisposed and/or that will compensate for loss of function caused by a genetic variant. A study by the Government Accountability Office [8] failed to find support for these claims; instead, this study found that the advice offered usually consists of only standard sensible dietary suggestions and lifestyle recommendations. The research community insists that current work in nutrigenomics is merely the tip of the iceberg and that it is still premature to determine the validity and utility of such testing. For example, to decrease blood pressure and the risk of cardiovascular disease, diabetes, and certain cancers, patients should be encouraged to follow current evidence-based guidelines with regard to everyday eating and to consume a balanced diet-one containing a colorful and plentiful variety of vegetables and fruits; moderate amounts of lean animal and/or plant proteins, healthy fats, and whole grains; and appropriate calcium sources. Patients should also be encouraged to avoid consuming too many calories and to cultivate an emotionally healthy approach to eating. At the present time, personalized advice on how to accomplish these goals will be more helpful to patients than personalized genomic test results.
Daunorubicin is widely distributed in tissues but does not cross the blood brain barrier treatment medical abbreviation order 200mg sustiva fast delivery. It is metabolized to daunorubicinol which is the major active metabolite and aglycones (inactive) medicine mart cheap sustiva 200mg with amex. The major route of elimination is through the bile (40%) with additional elimination through the urine medicine and manicures effective 200mg sustiva. Dosages should be reduced in patients with liver dysfunction (bilirubin > 1/2 mg/dL) or renal dysfunction (creatinine > 3 mg/dL) treatment for 6mm kidney stone discount sustiva 600mg on-line. Formulation and stability: Daunorubicin is supplied in vials containing 20 mg of reddish colored lyophilized powder and 100 mg of mannitol. Each vial can be reconstituted with 4 ml of sterile water for injection to give a final concentration of 5 mg/ml. Reconstituted solutions are stable for 24 hours at room temperature and 48 hours if refrigerated. Acute toxicity may take the form of arrhythmias, heart block or pericarditis and may be fatal. The chronic form of cardiotoxicity is related to total cumulative dose and is characterized by heart failure. Mediastinal radiotherapy and/or other cardiotoxic drugs may increase the risk of cardiotoxicity. Other toxicities include nausea and vomiting, mucositis, alopecia, diarrhea and red discoloration of the urine and other body fluids. Absorption of etoposide is approximately 30-40% and is highly variable and somewhat dosedependent. It is extensively bound to serum proteins and is metabolized in the liver, including cytochrome P450 3A metabolism to several moieties that include a reactive oxidized species. Etoposide and its metabolites are excreted mainly in the urine with a smaller amount excreted in the feces. Dosage adjustments should be considered in patients with liver dysfunction, kidney dysfunction or hypoalbuminemia. Formulation and stability: Etoposide is available in multi-dose vials containing 100mg, 150mg, 500mg and 1000mg of etoposide as a 20mg/ml solution and 30% alcohol. Nausea and vomiting (usually of low to moderate severity), diarrhea, mucositis (particularly with high doses), alopecia and anorexia are fairly common. Other side effects reported less commonly include hepatitis, fever and chills, anaphylaxis and peripheral neuropathy. Clofarabine also disrupts the mitochondrial membrane which results in the release of proteins, cytochrome C and apoptosis-inducing factor leading to cell death. Formulation and stability: Clofarabine is available in the parenteral form as a preservative-free solution that is 1 mg/mL. Supplier: the injection is commercially available, however for this study clofarabine will be supplied by Genzyme. Toxicity: the most common side effects are nausea, vomiting, diarrhea, headache, fever and pruritus. Also reported are pericardial effusion, tachycardia, hypotension, left ventricular systolic dysfunction, edema, flushing, hypertension, fatigue, anxiety, pain, dizziness, depression, irritability. Patients who receive clofarabine are at risk for tumor lysis syndrome and need to be monitored closely. The parent drug and metabolites are excreted primarily via hepatobiliary excretion with small amounts excreted in the urine. Dosage adjustment is recommended for patients with severe hepatic dysfunction (total bilirubin > 3. Formulation and stability: Mitoxantrone is available in multi-dose vials containing 20 mg, 25 mg or 30 mg of mitoxantrone as a dark blue, aqueous solution at a concentration of 2 mg/ml. Refrigeration may result in precipitation of mitoxantrone, which will re-dissolve upon warming to room temperature. These solutions are chemically stable for at least 7 days when stored at room temperature. Toxicity: the major dose-limiting toxicity of mitoxantrone is leukopenia with thrombocytopenia and anemia occurring much less frequently. Other side effects reported commonly include alopecia, diarrhea, headache, fever and stomatitis. Other side effects reported less commonly include elevated liver function tests, allergic reactions, seizures, jaundice and renal failure.
The advent of non-viable vectors with biodegradable substances and construction of mimetic systems will be of great value for solving the problems associated with the viral vectors treatment for shingles buy sustiva 600 mg visa. Nanotechnology tools symptoms bronchitis discount sustiva 200 mg free shipping, as well as new cell visualization and characterization tools treatment yeast uti order sustiva 200 mg with mastercard, will allow the advent of safer transfection techniques medications that raise blood sugar order sustiva 600mg free shipping. As there are several disciplines involved, advances in gene therapy depend on different technologies, which may be obtained only by multidisciplinary research. The collection of toxicological data is important to allow the clarification of the level of risk of different classes of viral vectors. The approval of the first medication intended for gene therapy opens new perspectives to obtain such essential data at a critical time for discussion about the safety of these vectors. Additionally, the increasing availability of new data will form the basis of future guidelines for production, marketing, clinical trials, and safety for these products. Detailed knowledge of the human genome, which has been achieved in the last few years, provide a rapid development tool and targets for gene therapy. A great impact is expected on human healthcare with this new knowledge, creating high expectations concerning the genesis of new products intended for the treatment of infectious diseases and tumors, which do not have alternative treatments. Safe manufacturing processes are already available, and the production capacity for these systems is easily accessible worldwide. Thus, it is possible to conclude that this therapy, still in its early phases, will cause a great impact on human health in the next few decades. The specific growth rate is limited by glutamine and inhibited by secreted ammonium and lactate (Equation A. The proposed mathematical modeling to fit the experimental data is based on Bree et al. The proposed model represents adequately the characteristic phenomena of the process. Wu1* Department of Pediatric Dentistry, College of Dentistry, University of Illinois at Chicago, Chicago, Illinois,1 and State Key Laboratory of Oral Diseases, Sichuan University, Chengdu, China2 Received 23 July 2010/Returned for modification 17 October 2010/Accepted 5 December 2010 Streptococcus mutans, the primary etiologic agent of dental caries, possesses a series of virulence factors associated with its cariogenicity. Alternatives to traditional antimicrobial treatment, agents selectively inhibiting the virulence factors without necessarily suppressing the resident oral species, are promising. The anticariogenic properties of tea have been suggested in experimental animals and humans. However, their effects on biofilm and cariogenic virulence factors of oral streptococci other than glucosyltransferases have not been well documented. Dental caries is a multifactorial infectious disease dependent on diet, nutrition, resident oral flora, and host response. Unlike most infectious diseases exhibiting classic virulence factors such as endotoxin (lipopolysaccharide), the etiology of dental caries is unique, being associated with the bacterial metabolism of carbohydrates, leading to prolonged periods of plaque acidification and demineralization of the tooth enamel. Bacterial virulence factors contributing to dental caries result in stable biofilm formation, stress tolerance. Despite the complexity of oral flora, oral streptococci, including Streptococcus mutans and Streptococcus sobrinus, have generally been considered the primary etiologic agents of dental caries (1, 38). These findings suggest that suppression of virulence-associated genes and enzymes could be appealing for the prevention of dental caries. Fluoride has long been proven to be an effective cariostatic agent (15, 17, 44) via the inhibition of demineralization and the enhancement of remineralization at the crystal surface, and the inhibition of bacterial activity (16). However, in the case of high bacterial challenge and/or xerostomia, fluoride alone is insufficient to prevent the progression of caries. In addition, adverse effects such as fluorosis have led to the limited use of fluoride for public health in many countries (16, 66). Therefore, the development of an alternative cariostatic agent with minimal side effects has been promising. Tea, an aqueous infusion of dried leaves of the plant Camellia sinensis (family: Theaceae), is the most popular beverage consumed by humans worldwide (18). Numerous in vitro and animal studies have demonstrated that polyphenols from 1229 Downloaded from aac. Subsequently, the wells were rinsed with deionized water until the blank wells appeared colorless; 200 l of 95% ethanol was added. The plates were shaken at room temperature for 30 min, and the absorbance at 595 nm was recorded.